
Читайте также:
|
H-C≡C-H?
A. 5σ- bonds
B. 4σ- and 1 π- bonds
C. 3σ- and 2π- bonds
D. 5 π- bonds

A. C3H4 B. C3H6 C. C2H2 D. C4H6
CH3-CH ═CH2 + HCl → ……………
A. 1,6 g B. 3,2 g C. 4,8 g D. 6,4 g
A. CnH2n+2
B. CnH2n
C. CnH2n-2
D. CnH2n-1
14. What is the name of the following compound?

15. 0,1mole of an alkane weighs 3 grams. What is the molecular formula of an alkane?
A. CH4 B. C2H6 C. C2H2 D. C2H4
16. What is the name of the product of the following reaction:
CH3-CH2-CH═CH2 + HBr →?
A. 1-bromobutane
B. 1-bromo-1-butene
C. 2-bromobutane
D. butane
17. Which one of the following is an isomer of CH3-CH2-CH2-CH3?
A. CH3-CH3
B. CH2═ CH-CH-CH3
C. CH3-CH-CH3
|
CH3
D. CH3-CH═CH-CH3
18. Which one of the followings does not react with ethylene?
A. Hydrogen
B. Chlorine
C. Water
D. Methane
19. Calculate mass of acetylene produced from 6,4 grams of calcium carbide according to the following reaction: CaC2 + 2H2O = Ca(OH)2 + C2H2?
(Ca: 40, C:12, H:1, O:16)
A. 2,6 g B. 1,3 g C. 3,9 g D. 5,2 g
20. In which pair of compounds there are homologous?
A. CH4 and CH≡CH
B. CH≡CH and CH3-CH3
C. CH2═CH2 and CH3-CH ═ CH2
D. CH≡CH and CH2═CH2
21. Monohydric alcohols do not react with
22. Which one of the following can react with each calcium, sodium hydroxide and ethanol?
A. Propanoic acid B. Propanal
C. Methylpropionate D. Propanol
23. The product of reaction between aldehyde and hydrogen is
A. ester B. alcohol
C. ether D. carboxylic acid
24. What is X in the following scheme 1-chlorobutane → X → 2-butanol
A. butane B. 2-chlorobutane
C. 1-butene D. 1-butanol
25. Which one of the following is an isomer of ethanol?
A. CH3OH B. CH3-O-CH3
A. FeCl3 B. [Ag(NH3)2]OH C. BaCl2 D. NaOH
27. Oxidation of which compounds give ketones?
A. Primary alcohols B. Secondary alcohols
C. Tertiary alcohols D. Ethers
28. Which one of the following is not a monosaccharide?
A. Fructose B. Glucose C. Sucrose D. Galactose
29. Oxidation of methanol produces
30. Qualitative reaction for polyhydric alcohols is reaction with
A. H2 B. [Ag(NH3)2]OH C. Fe D. Cu(OH)2
31 Which one of the following is an isomer of 1-butanol?
A. Propanol B. 2-butanol C. Butanal D. Butanone
32. How many grams of acetaldehyde can be prepared from 2,6 grams of acetylene by it’s hydration? (C:12, O:16, H:1)
33. Which one of the following is formic acid
A. CH3CHO B. CH3OH C. CH3CH2COOH D. HCOOH
34. Which alcohol can be produced by the fermentation of glucose?
A. Methanol B. Ethanol C. Propanol D. Butanol
35. Methanol does not react with
A. Ag B. K C. CuO D. O2
36. Reaction of monobromoalkane with aqueous base solution produces
A. alkane B. alkene C. alcohol D. aldehyde
37 Which ones are organic compounds?
1. C2H2 2. CaCO3 3. C2H5OH 4. CO 5 C2H5NH2
A. 1,2,4
B. 1,3,5
C. 2,3,4
D. 2,4,5
38. Which ones are homologous?
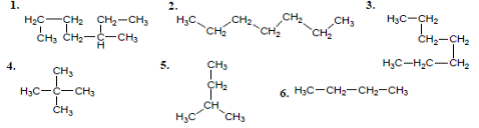
A. 1 and 3 B. 2 and 6 C. 2 and 4 D. 4 and 5
39 Name of the following hydrocarbon is

40 2-chloropropane can be produced by the reaction of
A. propene with Cl2
B. propene with HCl
C. propyne with HCl
D. cyclopropane with HCl
41 Benzene can react with
1. CH≡CH 2. Br2 3. H2 4. NaOH 5. O2
A. 1,2
B. 1,3,5
C. 4,5
D. 2,3,5
42. Compounds X and Y in the following scheme are: 
43 An organic compound contains 7,69% of hydrogen and 92,31% of carbon by mass. What is the molecular formula of a compound?
A. C6H10
B. C6H12
C. C4H8
D. C6H6
44. Phenol does not react with
1) sodium hydroxide 2) sodium 3) hydrochloric acid 4) bromine water
45. Which one is not an alcohol?
1) 2-butanol 2) ethanol 3) toluene 4) isopropanol
46. Which compounds have general formula CnH2n-2?
1) alkenes and alkynes 2) alkenes and alkadienes
3) alkynes and alkadienes 4) alkadienes and aromatic hydrocarbons (arenes)
47. Benzene can be prepared by trimerization of which hydrocarbon at the presence of carbon?
1) ethane 2) ethylene 3) acetylene 4) propene
48. Which statement is WRONG about alcohols
1) Alcohols can react with oxygen
2) Alcohols can undergo intermolecular dehydration reaction
3) Alcohols can react with bases
4) Alcohols can undergo intramolecular dehydration reaction
49. Qualitative reaction of aldehydes:
1) reduction by hydrogen to alcohols
2) oxidation to carboxylic acids with copper (II) hydroxide solution when heated with formation of red precipitate
3) reaction with bromine water with formation of white precipitate
4) reaction with alcohols with formation of esters
50. How many sigma σ-bonds in propyne molecule?
1) 6 2) 7 3) 8 4) 5
51. Hydrolysis of fats give carboxylic acid and
1) glycerol 2) formaline 3) phenol 4) starch
52. In reaction CH4 → X → CH3CHO, what is X?
1) ethane 2) ethylene 3) ethyne 4) ethanol
53. How many kJ of heat will be produced by combustion of 100g of benzene by following reaction C6H6 + 7,5O2 → 6CO2 + 3H2O + 3264 Kj
1) 2092 kJ 2) 8368 kJ 3) 6276 kJ 4) 4185 kJ
54. Qualitative reaction for phenol is formation of white precipitate in the reaction with
A. nitric acid B. metallic sodium C. bromine water D. hydrogenbromide
55. Esterification is a reaction of ester formation between
A. carboxylic acid and aldehyde B. carboxylic acid and alcohol
C. carboxylic acid and phenol D. two alcohol molecules
56. How many σ-bonds are in methanal?
A. 1 B. 2 C. 3 D.4
57. Which one is toxic for our organism?
A. glucose B. fructose C. ethanol D. galactose
58. What is X C2H2 → X → CH3COOH?
A. ethanol B. ethanal C. ethylene D. ethane
59. How many kJ of heat will be produced by combustion of 30 liters of acetylen by following reaction C2H2 + 2,5O2 → 2CO2 + H2O + 1300 kJ
1) 870,5 kJ 2) 3482 kJ 3) 1741 kJ 4) 4352,5 kJ
60 Which one is methyl radical?
A. CH3- B. C2H5- C. C3H7- D. C4H9-
61 Methylacetate and propanoic acid are
A. homologous
B. functional isomers
C. geometrical isomers
D. the same compound
62 Which one of the following can react with both water and acidic potassium permanganate solution?
A. CH4 B. C2H4 C. C6H6 D. C6H5CH3
63 Acetylene can be produced by the reaction of which of the following with alcohol base solution?
A. chloroethane
B. 1,1-dichloroethane
C. 1,1,2,2-tetrachloroethane
D. Ethylene
64 Ethylformate can be formed by the reaction of
65 What is X in the following scheme X+ HBr → CH3Br + H2O
A. Methane
B. Chloromethane
C. Methanal
D. Methnol
66 How many grams of acetaldehyde can be prepared from 2,6 grams of acetylene by it’s hydration? (C:12, O:16, H:1)
67. Weak acidic properties of alcohols and phenol are shown in the reaction with:
A. halogens B. aldehydes C. sodium D. inorganic acid
68. Organic compounds containing –CHO group show properties of:
A. alcohols B. carboxylic acids C. aldehydes D. esters
69. Which statements about carboxylic acids are correct?
70. Which one of the followings can show properties of both aldehydes and carboxylic acids?
A. Glucose
B. Acetaldehyde
C. Formic acid
D. Diethylether
71 Which one is product of polymerization of propene?

Дата добавления: 2015-10-30; просмотров: 122 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| А. (письменно) | | | Choose correct answers |