
Читайте также:
|
Glomerular growth may occur due to increase in any of its cellular components. These alterations in response to either the initial injury or to a decrease in remaining renal mass after initial injury contribute to maintaining renal function in the short term. However, in the long term these adaptive changes promote matrix accumulation and thereby renal malfunction.
The glomerular endothelial cell has a long half-life, approximately 100 days. Endothelial cell apoptosis as well as proliferation and migration occur in several animal models of glomerular injury. The balance between endothelial cell growth and death appear to be crucial for determining whether healing or scarring will result after injury37. Normally, endothelial cells inhibit smooth muscle cell migration and proliferation. Endothelial cells produce numerous factors that modulate mesangial or vascular smooth muscle cell growth (e.g., vascular endothelial growth factor, VEGF; nitric oxide; endothelin and PDGF)18. Some endothelial-derived factors act in synergy with heparin-like substances. VEGF is an endothelial cell-specific mitogen that within the kidney originates largely from epithelial cells, and may mediate physiologic and pathologic angiogenesis. Recent evidence implicates VEGF as a necessary survival factor for cells in vivo (abstract; Suga et al, J Am Soc Nephrol 10:561A, 1999). Endothelin, a powerful vasoconstrictor, is released from endothelial cells in response to a variety of stimuli and also after injury38. Endothelin results in the adaptive responses of hyperplasia, hypertrophy and increased matrix in mesangial cells in culture18,38. Plasminogen activator-inhibitor-1, PAI-1, which is produced by endothelial cells among other sources, has been linked to not only thrombosis, but also fibrosis through its effects to inhibit matrix degradation39. PAI-1 induction occurs in response to several growth factors and cytokines that play key roles in renal growth and fibrotic responses, including TGF- 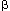 and angiotensin. These data indicate that PAI-1 may be an additional target for antifibrotic therapies.
and angiotensin. These data indicate that PAI-1 may be an additional target for antifibrotic therapies.
The glomerular visceral epithelial cell is part of the capillary wall barrier, and produces ECM. Glomerular visceral epithelial cells have limited capacity for hyperplasia. The exciting recent discoveries of specific cyclin-dependent kinase inhibitors expressed in mature, but not immature glomerular visceral epithelial cells suggest a possible mechanism for this limitation of this highly specialized cell40. Manipulations of one of these inhibitors, p27Kip-1, in experimental mice models illustrated that abnormal glomerular visceral epithelial cell proliferation, whether too little or too much, resulted in augmented glomerular injury40. Epithelial cells frequently are detached from the underlying basement membrane in settings of glomerular hypertrophy and sclerosis, perhaps due to their limited growth. These epithelial cell defects are postulated to play an important role in adhesions and hyalinosis, leading to progressive scarring41. Glomerular visceral epithelial cells also interact with other glomerular cells and modulate cell growth and matrix synthesis/degradation by elaboration of growth factors and cytokines, such as heparan (inhibits mesangial cell growth) and VEGF. Injured glomerular visceral epithelial cells also change phenotype. The Wilms' tumor gene WT-1, normally expressed in glomerular visceral epithelial cells, is decreased or lost in injured glomerular visceral epithelial cells in the severe collapsing form of FSGS, a dedifferentiation phenotype42. WT-1 has been implicated in a wide spectrum of functions in the kidney, including renal development, glomerular permselectivity and sclerosis.
The mesangial cell responds to many growth factors by proliferation and increased ECM synthesis. After initial injury, the activated mesangial cell changes phenotype, expressing fibroblast-like myosin43. This wound healing phenotype is associated with increased matrix generation.  -actin expression is also increased after injury, along with increased PDGF-B, recapitulating their developmental patterns of expressions in mesangial cells. Ang II induces mesangial cell hypertrophy and increases matrix production from mesangial cells in vitro. TGF-
-actin expression is also increased after injury, along with increased PDGF-B, recapitulating their developmental patterns of expressions in mesangial cells. Ang II induces mesangial cell hypertrophy and increases matrix production from mesangial cells in vitro. TGF- 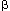 increases both collagen and proteoglycan production by mesangial cells, and depending on the cell cycle stage, acts synergistically with other growth factors. bFGF may initiate mesangial cell injury response. Mesangial cells in culture release both interleukin-1 and a PDGF-like factor, and proliferate and produce ECM in response to these substances, suggesting both autocrine and paracrine regulation of mesangial cell proliferation18,44.
increases both collagen and proteoglycan production by mesangial cells, and depending on the cell cycle stage, acts synergistically with other growth factors. bFGF may initiate mesangial cell injury response. Mesangial cells in culture release both interleukin-1 and a PDGF-like factor, and proliferate and produce ECM in response to these substances, suggesting both autocrine and paracrine regulation of mesangial cell proliferation18,44.
Topof page
Дата добавления: 2015-10-29; просмотров: 156 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| FACTORS MEDIATING GROWTH | | | CELL PROLIFERATION VERSUS APOPTOSIS |