
Читайте также:
|
Adaptations to increase renal mass and function presumably occur in attempts to restore renal function towards normal after injury. Renal growth occurs by both hyperplasia (increase in cell number) and hypertrophy (increase in cell size). Increases in renal mass primarily reflect tubular growth. However, glomerular growth also occurs in response to some injuries or reduced nephron mass. The glomerular growth occurs primarily by lengthening of the capillaries without significant increase in diameter after loss of renal parenchyma or in children with reflux nephropathy27,28. In contrast, growth is accomplished by new capillary branching in experimental diabetes and in toxic nephropathy due to lithium29,30. With more extreme hemodynamic abnormalities, dilatation of capillaries may occur and contribute to increased glomerular size31.
So-called glomerular hypertrophy, that is an increase in glomerular size, represents both cellular hypertrophy and hyperplasia. Glomerular hypertrophy can be detected biochemically and morphologically by electron microscopy as early as 2 days after 5/6 nephrectomy. Increased glomerular volume at 2 days was attributable to increased glomerular visceral epithelial cell expansion, likely hypertrophy in view of this cell's limited mitogenic capacity (see below)32. Later increases in glomerular volume were contributed to by mesangial cell increase, likely both hypertrophy and hyperplasia. Hyperplasia predominated after 5/6 nephrectomy, whereas hypertrophy was the major growth response after uninephrectomy. The resulting glomerular growth was more marked after 5/6 nephrectomy, and was associated with later sclerosis.
The precise trigger(s) for initiation of growth response remain undetermined. Early work proposed that renal growth after uninephrectomy was merely in response to increased work load. In the early 1970s the concept of "renotropic" factor(s) was formulated as the alternative explanation based on observations of renal growth in normal animals connected by parabiosis with an anephric partner, resurrecting observations made by Sacerdotti over a hundred years ago14,33. Most recently, a possible quantitative trait locus (QTL) linked to genetic modulation of the compensatory growth response has been identified in the mouse on chromosome 11. This marker maps near the genes for ACE, growth hormone and neural growth factor receptor (NGFR), all of which have known influences on growth responses in the kidney34. Hypertrophy of remnant glomeruli was also postulated to be the consequence of the abnormal hemodynamics developing within these glomeruli or merely represent engorgement. However, the increased growth in the remnant kidney model in the rat occurred before increases in single nephron GFR14.
Altered gene expressions in pathophysiologic settings implicate many factors in glomerular growth and enhanced ECM release, including the following: platelet derived growth factor (PDGF), transforming growth factor- 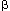 (TGF-
(TGF- 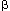 ), TGF-
), TGF-  , IGF-1, growth hormone, epidermal growth factor, interleukins 1 and 6, tumor necrosis factor-
, IGF-1, growth hormone, epidermal growth factor, interleukins 1 and 6, tumor necrosis factor-  (TNF-
(TNF-  ), Ang II, hepatocyte growth factor and its receptor c-myc, and endothelin. Several growth factors are now considered to play key causal roles in glomerulosclerosis, including Ang II, PDGF and TGF-
), Ang II, hepatocyte growth factor and its receptor c-myc, and endothelin. Several growth factors are now considered to play key causal roles in glomerulosclerosis, including Ang II, PDGF and TGF- 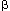 , based on results of infusion, transfection and inhibition of these factors. Studies in human renal diseases have begun to map some of these key growth factors, showing up-regulation in some settings. Interpretation must take into account that a given growth promoter may affect different cells differently, inducing proliferation, hypertrophy or even inhibiting growth, depending on the host cells' milieu, genotype and phenotype.
, based on results of infusion, transfection and inhibition of these factors. Studies in human renal diseases have begun to map some of these key growth factors, showing up-regulation in some settings. Interpretation must take into account that a given growth promoter may affect different cells differently, inducing proliferation, hypertrophy or even inhibiting growth, depending on the host cells' milieu, genotype and phenotype.
Topof page
Дата добавления: 2015-10-29; просмотров: 121 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| GLOMERULAR HYPERTROPHY IN EXPERIMENTAL MODELS | | | CELL-SPECIFIC GROWTH RESPONSES |