
Читайте также:
|
a. Substances react together producing new substances. The activity which changes the substance into a new material is called chemical reaction.
b. An atom is the unit of the substance which undergoes a chemical reaction.
c. An atom that has less than 8 electrons in the last energy level is active.
It reacts with atoms of other elements to complete the last energy level with 8 electrons.
An example:
sodium atoms are active because each has an electron in the last energy level. In a chemical reaction sodium gives this electrons to atoms of another element & end up with 8 electrons in the last energy level.
d. Inert gases such as Argon 18Ar has 8 electrons in the last energy level. They are stable & don’t react with other substances.

I. Fill in the blanks:
| Name | Symbol | No. of protons | No. of neutrons | Electronic configuration |
| Hydrogen | 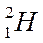
| |||

| ||||
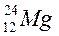
| ||||
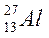
| ||||
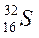
| ||||

|
II. Give reasons:
1. The atom is electrically neutral.
………………………………………………………………………………………………………………………………………………………………………………………………………….
2. The mass number is greater than the atomic number.
…………………………………………………………………………………………………………………………………………………………………………………………………………
3. The 3rd energy level in the atom contains 18 electrons.
………………………………………………………………………………………………………………………………………………………………………………………………………….
4. The equation 2n2 isn’t applied to the 4th energy level.
………………………………………………………………………………………………………………………………………………………………………………………………………….
5. 10Ne doesn’t go into chemical reactions.
……………………………………………………………………………………………………………………………………………………………………………………………………….
6. The mass of the atom is concentrated in the nucleus.
………………………………………………………………………………………………………………………………………………………………………………………………………….
III. Write the scientific term:
| 1. The number of protons in the atom. | |
| 2. The sum of no. of protons & neutrons in the nucleus. | |
| 3. The basic unit of matter that takes part in a chemical reaction. | |
| 4. Negatively charged particles that revolves around the nucleus. |
IV. Choose the right answer:
1. The second energy level in 7N contains………….electrons.
(2 – 3 – 5)
2. The chemical activity of the element depends on the number of …………………….
(electrons in the outer level – protons – neutrons)
3. All the following elements are chemically active except…………………
(1H – 6C – 18Ar)
V. Figure………………………is an excited atom while, figure…………………is for the atom in the ground state.
 | |||||
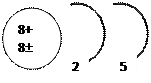 |  | ||||
Figure A Figure B
Дата добавления: 2015-10-28; просмотров: 115 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| The atomic number is the number of protons in an atom. | | | VOCABULARY EXERCISES |