
Читайте также:
|
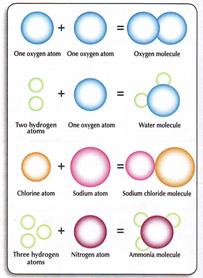 If the atoms in the molecule are similar, this substance is an element. An element is the simplest form of a substance.
If the atoms in the molecule are similar, this substance is an element. An element is the simplest form of a substance.
A compound results from the combination of 2 or more different elements.

| Gaseous elements | A molecule consists of 1 atom | Inert gases (noble gases) | Helium, xenon, neon argon, krypton, radon |
| A molecule consists of 2 atoms | Oxygen Chlorine Fluorine Hydrogen Nitrogen | ||
| Liquid elements | Bromine | ||
| A molecule consists of one atom | Mercury | ||
| Solid elements | Magnesium,aluminium, iron & carbon | ||
| Points of comparison | Structure of the molecule | Examples |

I. Explain why:
1. The table salt dissolved in water disappears.
………………………………………………………………………………………………………………………………………………………………………………………………………………
2. The volume of a mixture of water & alcohol is less than the sum of their volumes before being mixed.
………………………………………………………………………………………………………………………………………………………………………………………………………………
3. The smell of perfume spreads in the room.
……………………………………………………………………………………………………………………………………………………………………………………………………………………
II. Write the scientific term:
| 1. The simplest form of matter that couldn’t be analyzed. | |
| 2. The substance that consists of combination of different elements. | |
| 3. The spaces among molecules. | |
| 4. The smallest part of matter that can exist freely. |
III. Match the figure with the following molecules:
1. Hydrogen molecule 2. Water molecule 3. Helium molecule
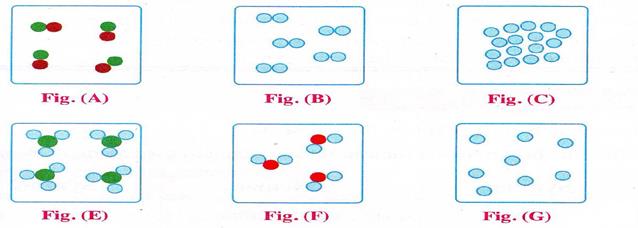 4. Sodium molecule 5. Ammonia molecule 6. Iron molecule
4. Sodium molecule 5. Ammonia molecule 6. Iron molecule
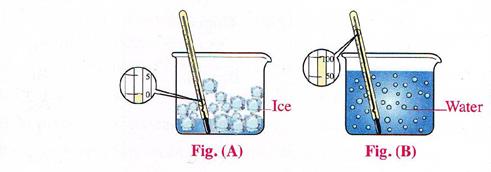
IV. Match the figure with the process:
a. Figure………………………………..indicates the vaporization process.
b. Figure……………………………….indicates the melting process.

1. Chemists use symbols to express elements.
2. Some symbols come from the Italian name of the element (Latin is an old European language).
3. The symbol is a capital letter, if the symbol consists of 2 letters only the first one is a capital letter.
| Element | Atom symbol | Element | Atom symbol |
| Lithium | Li | Hydrogen | H |
| Potassium | K | Oxygen | O |
| Sodium | Na | Nitrogen | N |
| Calcium | Ca | Fluorine | F |
| Magnesium | Mg | Chlorine | Cl |
| Aluminium | Al | Bromine | Br |
| Zinc | Zn | Iodine | I |
| Iron | Fe | Helium | He |
| Lead | Pb | Argon | Ar |
| Copper | Cu | Sulphur | S |
| Mercury | Hg | Phosphorous | P |
| Silver | Ag | Carbon | C |
| Gold | Au | Silicon | Si |
 Scientists found that atoms consist
Scientists found that atoms consist
of a nucleus & electrons.
| The nucleus | The electrons |
| 1. The central core of the atom. 2. Consists of protons & neutrons. 3. Carries positive charges. 4. It has most of the mass of the atom. | 1. They revolve in orbits around the nucleus 2. Their charge is negative 3. Their masses are small (negligible) |
| Protons | Neutron |
| 1. Positively charged | 1. Neutral (carry no charge) |
| 2. Exist in the nucleus | 2. Exist in the nucleus |
| 3. The mass of a proton almost equals that of a neutron, together protons & neutrons contain most of the mass of the atom. |
The number of protons in the atom of an element is unique & characterizes the element.
Дата добавления: 2015-10-28; просмотров: 145 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| VI. Thermal conduction | | | The atomic number is the number of protons in an atom. |