
Читайте также:
|
The mass number is the sum of protons & neutrons in an atom.
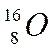 Atomic number (written at the left side below the symbol is 8) = 8 protons.
Atomic number (written at the left side below the symbol is 8) = 8 protons.
Mass number (written at the left side above the symbol is 16)
No. of neutrons = 16 – 8 = 8
Nitrogen 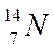
Atomic number = no. of protons = 7
Mass number = protons + neutrons = 14
Number of neutrons = 14 - 7 = 7
Complete the following table:
| Element symbol | Atomic number | Mass number | Number of protons | Number of neutrons |

| ||||
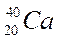
| ||||
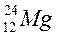
| ||||
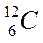
| ||||
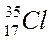
| ||||
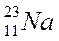
|
An atom is electrically neutral because the number of protons = the number of electrons. The positive charge of the proton is opposite to that of the electrons.
Electrons revolve around the nucleus in orbits called energy levels.
a. 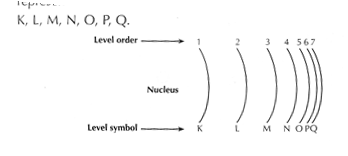 Energy levels are places around the nucleus where electrons exist.
Energy levels are places around the nucleus where electrons exist.
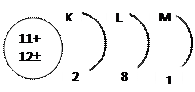
b. There are 7 energy levels represented from the nearest to the nucleus to the farthest by the letters K, L, M, N, O, P, Q.
c. The energy of the level increases by the increase of the distance from the nucleus.
d. Each level is saturated (completely filled) with a certain number of electrons.
e. The number of electros which saturate energy levels 1-4 is calculated by the rule 2n2
Where n is the number of the energy level.
| No. of energy level | It symbol | The number of electrons that saturate the levels |
| K | 2 (1)2 = 2 | |
| L | 2 (2)2 = 8 | |
| M | 2 (3)2 = 18 | |
| N | 2 (4)2 = 32 | |
| O | 32 electrons. The equation isn’t applied because a number of electrons larger than 32 makes the atom unstable. | |
| P | ||
| Q |
f. 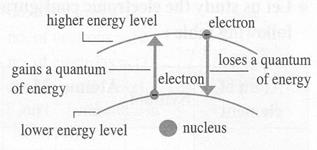 When an electron gains a quantum of energy, it moves to a higher energy level. The atom is excited by gaining energy.
When an electron gains a quantum of energy, it moves to a higher energy level. The atom is excited by gaining energy.
g. The excited atom loses the quantum of energy & electrons return to the original level (ground state)
Distributing electrons in energy levels electronic configuration.
| Mass number symbol atomic | Electronic configuration | ||||||||
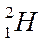
|
 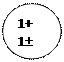
| ||||||||
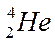
|
 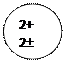
| ||||||||
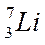
|
 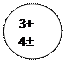
| ||||||||
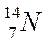
|
| ||||||||
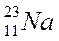
| 
|
Complete the following table:
|
| Mass number Atomic number | Electronic configuration |
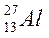
| |
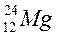
| |
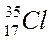
| |
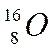
|
Дата добавления: 2015-10-28; просмотров: 137 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| A molecule consists of atoms. | | | The atom is the basic unit of matter which goes into in a chemical reaction |