
|
Читайте также: |
Laboratory work 8. Carbohydrate metabolism
Food substances (starch, glucose, fructose, sucrose, lactose, maltose) are carbohydrate source of organism. Starch is the storage form of glucose in plants; lactose is found in milk, glucose and fructose - in fruits and honey, maltose is present in products that contains partially hydrolyzed starch, such as malt. Normal intake of carbohydrates is 400-500 g/day. Carbohydrates are the main source of energy for human body.
Polysaccharides and disaccharides are digested in the gastrointestinal tract. Starch is partially digested in the mouth by α-amylase of saliva, splitting α-1,4- glycosidic bonds. Dextrin and maltose are formed. Pancreatic amylase hydrolyzes starch in the upper part of the small intestine. Maltose and isomaltose are formed. Maltose, isomaltose, sucrose and lactose are hydrolyzed by glycosidases on the surface of cells of the small intestine to form monomers. The resulting monosaccharides are absorbed in the intestine, enter the bloodstream and then come to the liver through the portal vein. Part of glucose passing through the liver, is carried by the blood throughout the body, but the main part is deposited in the liver as glycogen. When blood glucose level is decreased, glycogen is broken down to glucose. Despite continuous glucose consumption by all cells in the body its concentration in the blood is maintained at a steady level 3.3-6.1 mmol/l. Constancy of the blood glucose concentration is regulated by the action on the liver through the central nervous system and the endocrine system (mainly - pancreas, pituitary and adrenal glands).
Hyper- or hypoglycemia (high or low blood glucose levels) are characteristic of some diseases. Glucose is found in very small quantities in urine of healthy human. It cannot be found by common qualitative reactions. The phenomenon when glucose in urine is determined by common chemical methods is called glycosuria. Glycosuria may be a consequence of hyperglycemia and to be associated with decreased insulin production, hyperfunction of adrenal glands, pituitary gland, thyroid gland, as well as a one-time intake of large amounts of sugar. Therefore, the determination of glucose in biological fluids is important for clinical diagnostics.
Quantitative identifying of amylase activity in blood serum.
Urine and blood serum of healthy people have low amylase activity in comparison with saliva. Identifying amylase activity in urine and blood serum is used in clinical practice while diagnosing thepancreatic gland diseases. Normally alpha-amylase activity in blood is 28-100 IU/L, pancreatic amylase activity – 0-50 IU/L. Alpha-amylase activity in urine is 1-17 IU/h. In acute pancreatitis, the enzyme activity in blood and urine is increased 10-30 times, in chronic pancreatitis, pancreatic cancer in 3-5 times. Decreased kidney function (renal disorder) as well as some blood disorders cause enzyme activity increase only in blood but not in urine.
Amylase enzyme catalyzes the hydrolysis of α-1,4-glycosidic bonds of starch and glycogen. The method is based on colorimetric estimation of starch concentration before and after enzymatic hydrolysis according to coloring in reaction with Lugol’s reagent. Do the work according to the Table.
Table. Identifying amylase activity.
| Chemical reagents | Volume, ml | |
| Test tube 1 - experimental | Test tube 2 - control | |
| Starch solution | 1,0 | 1,0 |
| Blood serum | 0,02 | - |
| Incubate at 370 C for 5 minutes, then add: | ||
| Lugol’s reagent | 1,0 | 1,0 |
| Distilled water | 8,0 | 8,0 |
| Blood serum | - | 0,02 |
Photometer both of the solutions using photoelectric colorimeter at 630 nm (the thickness of the cuvette is 10 mm) against water.
The amylase activity is counted using the formula:
((Dc – De)/Dc)×200,
where Dc is optical density of the control test, De – of the experiment test.
Express diagnostics of carbohydrate metabolism pathologies.
All experiments should be done with urine samples 1 and 2.
2.1. Trommer’s tes.
Pour to 1 ml of urine in the test tube and add 1 ml of 10% sodium hydroxide solution. Then add 1% copper sulfate solution drop by drop till the precipitate of copper hydroxide (II) appears. The appearance of red coloring when heated testifies about the presence of glucose in urine.
Gaines test.
Pour 3-4 ml of Gaines reagent (prepared from copper sulfate, sodium hydroxide and glycerol) in the test tube and add 1 ml of urine. Heat the upper part of the solution until boiling of the reaction mixture.
2.3. Selivanov’s test.
The method is based on the conversion of fructose when heated and in the presence of hydrochloric acid into hydroxymethyl furfural which condenses with resorcinol (0.05% solution in 20% hydrochloric acid, Selivanov’s reagent), forming the compound of red color.
Pour 1 ml of resorcinol solution into 2 test tubes. Add 2 ml of urine and heat the test tube in the water bath till boiling. If fructose is present in the urine there will be bright red coloring.
2.4. Enzymatic method of semi-quantitative identification of glucose in urine with the help of "GLUCOPHAN" test strip.
"GLUCOPHAN" test strip has one reagent pad of light-yellow color, soaked with the solutions of enzymes glucose oxidase, peroxidase and coloring agent (benzidine derivative) solution. Glucose oxydase is a flavoprotein, the prosthetic group of which is FAD. It catalyzes the transfer of two atoms of hydrogen from glucose to aerial oxygen. The formed hydrogen peroxide is degraded by peroxidase enzyme, due to which the coloring agent is oxidizes. The change of the coloring agent color when it oxidizes testifies about the presence of glucose in the urine. This method allows us to identify the concentration of glucose in urine approximately from 0.1 up to 2%.
Pour a little amount of experimental urine into a glass. Dip "GLUCOPHAN" test strip into the experimental urine to soak the yellow reagent pad completely. Immediately remove the strip from the liquid, wait for 1 minute. Compare the color of the reagent pad with the colored scale. If glucose is absent in the urine the reagent pad color does not change. Find the most matching colors on the reagent pad and on the colored scale to identify the concentration of glucose approximately.
Test Questions
1. What are the optimal conditions for pancreatic amylase functioning?
2. What products will be formed from starch in the presence of pancreatic juice (in vitro)?
3. How can the presence of starch conversion products be identified in a sample?
4. What functional group of glucose molecule causes Trommer’s positive test?
5. What can be cause of hyperglycemia?
6. What diseases or physical conditions cause glucosuria?
7. What are causes of fructosuria?
Laboratory work 9. Intermediate products of carbohydrate metabolism
Quantification of pyruvic acid in urine
Pyruvic acidis one of the intermediate products of carbohydrate metabolism. Under anaerobic conditions (hypoxia) it is reduced into lactic acid, and under aerobic ones it undergoes oxidative decarboxylation and is converted into acetyl-coenzyme A. Pyruvic acidis one of the main sources for gluconeogenesis. As a result of high rate of the conversion pyruvic acidis present in tissues and biological fluids in little amounts. In blood its concentration is 0.5-1 mg/100 ml. The concentration of pyruvate in urine is normally 2 mg/100 ml. It daily excretion with urine is 10-25 mg.
The most rapid increase of pyruvate concentration in blood and as a consequence in urine is noticed after muscular work and in vitamin В1 deficiency. This phenomenon is also noticed in hepatic disorders, diabetes, cardiac decompensation, toxicosis, etc.
The method principle: pyruvate and 2,4-dinitrophenylhydrazine form a colored compound 2,4-dinitrophenylhydrazone of pyruvate. It is extracted from the reaction mixture by toluene. When alkali alcohol solution is added, it gradually turns red-orange; the optical density of the solution is directly proportional to the concentration of pyruvic acid.
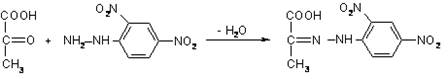
pyruvic acid 2,4-dinitrophenylhydrazine dinitrophenylhydrazone of pyruvic acid
Take 3 test tubes. Pour 1 ml of urine diluted 3 times into the one tube (experimental sample), 1 ml of pyruvic acid standard solution into the second tube (standard sample), and 1 ml of water into the third tube (control sample).
Add 0,5 ml of 0,1% 2,4- dinitrophenylhydrazine solution to all samples, mix. After 5 min add 2,5 ml of water-saturated toluene into each test tube. After shaking for 1 minute leave the solutions for water and toluene demixing.
Take a 1 ml sample from the upper toluene layer with a dry graduated pipette from each test tube and place it into dry test tubes, add 3 ml of potassium hydroxide alcohol solution. Wait for 10 minutes.
Use the control sample for photoelectric colorimeter calibration. Measure the optical density of the standard sample and experimental sample by the wave length of 400-415 nm using photoelectric colorimeter.
The calculation is made according to the formula:
Х = De*50*3/Ds, where:
Х is the concentration of pyruvate in urine, mg|100 ml
De is optical density of the experimental sample,
Ds is optical density of the standard sample,
50 is the concentration of pyruvic acid standard solution, mg|100 ml,
3 – degree of urine dilution.
Дата добавления: 2015-10-26; просмотров: 203 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Comparison of redox-potentials of riboflavin and methylene blue. | | | Key positions |