
|
Читайте также: |
Laboratory work 13. The end products of nitrogen metabolism
The main end product of protein metabolism is urea. The synthesis of urea occurs in the liver from ammonia. Normally, about 30 g of urea (0.5 mole) is excreted per day with the human urine. The urea increase in urine is observed on a protein-rich diet and due to diseases related to an enhanced breakdown of the body's own proteins (fever, hyperthyroidism, diabetes). A reduced urea excretion is observed on a protein-poor diet and due to kidney diseases when the urea excretion is hampered; in liver diseases characterized by the urea synthesis disorder; in acidosis when an excessive amount of ammonium salts is formed and as a result urea synthesis decreases.
Ammonium salts are formed as a result of the neutralization of acids, entering the body from the outside or formed inside in metabolic processes, by ammonia. 0.5-1.2 g of ammonia is excreted per day with the urine in the form of ammonium salts normally. The ammonia excretion with the urine is increased due to the diseases accompanied by acidosis, such as diabetes mellitus, when ketone bodies are formed and the concentration of phosphoric and sulfuric acids is increased due to an enhanced tissue proteolysis. The decreased amount of ammonia in urine is characteristic of kidney damage due to loss of the ability to obtain ammonia from glutamine.
Creatinine is one of the end products of protein metabolism. It is formed in the muscular tissue from creatine phosphate. The daily excretion reflects the muscular mass of the body. Creatine normally is practically absent in urea of adult person. It appears in pathologies as liver diseases, diabetes mellitus, hyperthyroidism, infectious diseases, or using a diet rich in creatine.
Biuret test to urea.
Put some urea crystals into a dry test tube and melt them in the flame. Continue heating until the melted mass starts to harden. Ammonia gets split from urea, and biuret is formed. Bring red water-wetted litmus paper to the opening of the test tube. Record the changing of paper color. Then cool the biuret obtained in the test tube, dissolve it in 0.5 ml of 10% sodium hydroxide solution and add 1-2 drops of 1% copper sulfate solution.
Quantitative determination of creatinine in urine by Jaffe color reaction (the method of Popper).
Creatinine interacting with picric acid forms creatinine picrate which converts to its tautomeric orange-red form in an alkaline medium. The color intensity is proportional to the concentration of creatinine.

The control sample. Mix 3 ml of a saturated solution of picric acid and 0.2 ml of 10% sodium hydroxide solution in a volumetric flask of 100 ml.
The standard sample. Mix 0.5 ml of the base standard solution of creatinine and 3 ml of a saturated picric acid solution in a volumetric flask of 100 ml. Shake the flask and add 0.2 ml of 10% sodium hydroxide solution.
The experimental sample. Put 0.5 ml of urine (sample 1) and 3 ml of a saturated solution of picric acid into a volumetric flask of 100 ml. Shake the flask and add 0.2 ml of 10% sodium hydroxide solution.
Leave flasks for 10 minutes at room temperature and then add distilled water until the total volume of the contents is 100 ml.
Measure the optical density of the standard and experimental samples with an electrophotocolorimeter in a cuvette of 1cm layer thickness at a 510-560 nm wavelength (green filter) against control.
Calculations are made according to the formula:
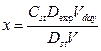 , where
, where
x is the creatinine mass in daily urine, mg;
Cst is the concentration of creatinine in the standard sample, mg;
Dexp is the optical density of the experimental sample;
Dst is the optical density of the standard sample;
Vday is the daily volume of urine;
V is the volume of urine taken for analysis.
Compare the obtained data with normal level of creatinine in daily urine: for men – 1-2 g/day (8.8-17.7 mmol/day), women – 0.8-1.8 g/day (7.1-15.9 mmol/day).
3. Quantitative determination of ammonia in urine according to Malfatti’s method.
Put 5 ml of urine (sample 2) into a flask, add 25 ml of distilled water, 2 drops of phenolphthalein and titrate with 0,1 n. solution of sodium hydroxide until a light pink coloring appears. Thus the neutralization of acidic substances contained in the urine is achieved. Add 2 ml of formalin. The mixture gets discolored due to the breakdown of ammonium salts and appearance of acid in the solution.
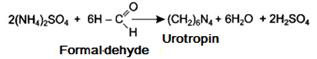
Then titrate the mixture with 0.1 n. NaOH solution until a pink coloring appears. Multiply the amount of the alkali (ml) used during the last titration by 0.0017 (titre of 0.1 n ammonia solution) to get ammonia mass for the urine volume under analysis. Estimate the amount of ammonia excreted with urine per day, if it daily volume is 1200 ml. Make a conclusion about the presence or absence of acidosis.
Tasks
1. Give the equations of the acetoacetic acid and 3-hydroxybutiric acid neutralization by ammonia.
2. Give the equation of the conversion of creatine into creatinine.
Дата добавления: 2015-10-26; просмотров: 372 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Задание 5. Выполняется самостоятельно. | | | Titration of acids of gastric contents. |