
Читайте также:
|
1- Proteins
2- Peptide bond
3- Dipeptide chain
4- Polypeptide chain
5- Nucleic acids
6- Casein proteins
7- Simple proteins
8- Associated proteins
9- Hemoglobin
10- Nucleotides
11- Ribose
12- Deoxyribose
13- DNA
14- RNA
Lesson (3) Water

à Water is the basis of life on our planet, it exists in different physical states (solid, liquid, gas). Water makes up:-
à 70% of the surface of earth
à (65% - 90%) of the weight of living organisms
The importance of water
It plays an important role in all vital processes within living organisms.
The molecular structure of water
àwater molecule H2O is composed of one oxygen atom and two hydrogen ones, these atoms are bound covalently (by covalent bond).
àIn water molecule, hydrogen carries molecular negative charge, while oxygen carries molecular positive charge. Thus, water molecule is polar molecule because it has positive and negative poles.

Fig. (10) The molecular structure of water
à Close water molecules are attracted to each other by relatively low electric attraction (as negative hydrogen atoms attract positive oxygen atoms in another molecules). This bond is called Hydrogen bond
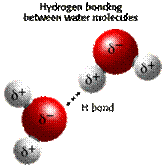
Fig. (11) Hydrogen bond diagram
à Water has unique properties because of its polarity and hydrogen bonds between its molecules

Water is a polar solvent
Water is from the best solvents and regarded as "the general solvent" due to the polarity of its molecules
Example:-
When sodium chloride NaCl dissolves in water, it produced positive sodium ions and negative chlorine ions. Thus, positive oxygen atoms of water attract negative chlorine ions, and negative hydrogen atoms attract positive sodium ions.
à All polar substances (substances containing ions) can dissolve in polar solvents such as water
Дата добавления: 2015-10-23; просмотров: 167 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Choose the correct answer | | | Importance of this property |