
|
Читайте также: |
Laboratory work 1
The analysis of proteins and amino acids
Proteins can be called the basic molecules of life. Proteins are high molecular nitrogen-containing organic compounds found in all living cells. The building blocks of proteins are the twenty naturally occurring amino acids. Amino acid residues are joined by peptide bonds. Proteins differ only in the number, the nature, and the sequential order of their constituent amino acids. These amino acids are liberated when proteins are hydrolyzed.
The solubility of proteins in an aqueous solution containing salts depends on two opposing effects on the one hand related to electrostatic interactions ("salting in") and other hydrophobic interactions (salting out).
A protein is denatured when its specific three-dimensional conformation is changed by breaking some bonds without breaking its primary structure. Denaturing agents are physical or chemical agents. The denaturation may be reversible or irreversible. It causes a total or partial loss of biological activity. This is an important property of protein.
Characteristic physical and chemical properties of proteins are high viscosity of the solutions, low ability to diffusion, swelling capacity, optical activity, mobility in an electric field, low osmotic pressure and high oncotic pressure. Protein molecules can not penetrate through the semipermeable membrane.
Qualitative analysis of amino acid mixtures by thing layer chromatography method.
Prepare chromatographic plate. Draw the straight line by the pencil at 1.5-2 cm from the border of chromatographic plate. Than draw 4 points on this line. At points 1, 2, 3 apply amino acids solutions with capillary, and to point 4 - the solution of amino acid mixture. Dry the chromatogram over a warm electric stove.
Put the chromatogram into a chromatogram chamber, at the bottom of which a mixture of butanol, acetic acid and water (15:3:7) is poured so that the solvent does not reach the starting line. Close the chamber with a cover glass and leave the chromatogram for some time.
The aqueous component of the solvent system binds to the plate porous layer and forms a stationary phase. The organic component that migrates along the plate is the mobile phase. When the migration of the solvent is upwards, it is referred to as ascending chromatography. As the solvent flows, it takes along with the unknown substances. The rate of the migration of molecules depends on their relative solubility in the stationary phase (aqueous) and mobile phase (organic).
When the solvent traverses the path from the bottom to up remove the chromatogram from the chamber, mark the front line of the solvent and dry the plate. Apply 0.5% solution of ninhydrin in acetone on the dried chromatogram in the spray chamber with the help of the pulverize. When the acetone evaporates, the chromatogram is placed over a warm electric stove for some min. The positions of amino acids on the chromatogram are identified as purple spots.
Calculate Rf (retardation factor) by the formula:
Rf = Хi/Х0,
where Х0 is the distance travelled by solvent (from the starting line to the front line of the solvent), Хi is the distance travelled by substance (from the starting line to the middle of corresponding spot).
The Rf value of each substance, characteristic of a given solvent system and paper, often helps for the identification of unknown. Comparing the values Rf of known amino acids and amino acids of the identifying mixture do the conclusion about the nature of amino acids in the mixture.
1. Draw the chromatogram.
2. Calculate Rf. Compare values.
Colour reactions of proteins.
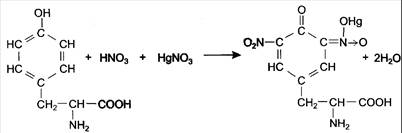
2.1. Millon’s reaction.
Millon's reagent consists of nitrate of mercury (I) and (II) in HNO3 with admixture of HNO2. The reaction of Millon's reagent with proteins is possible due to the presence of tyrosine residue in proteins.
Pour 0.5 ml protein solution and 0.5 ml of Millon's reagent in the test tube. A white precipitate is formed under the action of mercury salts and nitric acid, which are the part of Millon's reagent. Then heat the test tube.
Дата добавления: 2015-10-26; просмотров: 194 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| The order of modeling of measurements and analysis of its results | | | Digestion of fats. Influence of bile salts upon pancreatic lipase activity. |