
Читайте также:
|
4. Which method do you find:
- the easiest;
- the most complex;
- the most interesting;
- to be common;
- to be widespread.
Render the text.
Speaking
1. Using conversational gambits given in the Appendix 2 construct a dialogue “The best method of resolution”.
2. What my folks know: In pairs, talk about how much you think your family and friends know about enantiomers and how interested they are (or otherwise). Report on what your partner said.
| Level of interest | Level of knowledge | |
| Partner | ||
| Parents | ||
| Siblings | ||
| Grandparents | ||
| Best friend | ||
| Workmate | ||
| Other |
Read the following text. Discuss whether what they came up with is really so difficult, why the subject of the text may not be as easy as it seems. Share and compare your opinion with partners.
Optical Purity
Suppose we have just attempted to resolve a racemic mixture by one of the methods described in the previous section. How do we know that the two enantiomers we have obtained are pure? For example, how do we know that the (+) isomer is not contaminated by, say, 20% of the (-) isomer and vice versa? If we knew the value of [ɑ] for the pure material, [ɑ]max, we could easily determine the purity of our sample by measuring its rotation. For example, if [ɑ]max is +80° and our (+) enantiomer contains 20% of the (-) isomer, [ɑ] for the sample will be +48°. We define optical purity as

Assuming a linear relationship between [ɑ]max and concentration, which is true for most cases, the optical purity is equal to the percent excess of one enantiomer over the other:

But how do we determine the value of [a]max? It is plain that we have two related problems here; namely, what are the optical purities of our two samples and what is the value of [ɑ]max. If we solve one, the other is also solved. Several methods for solving these problems are known.
One of these methods involves the use of NMR. Suppose we have a nonracemic mixture of two enantiomers and wish to know the proportions. We convert the mixture into a mixture of diastereomers with an optically pure reagent and look at the NMR spectrum of the resulting mixture, for example,
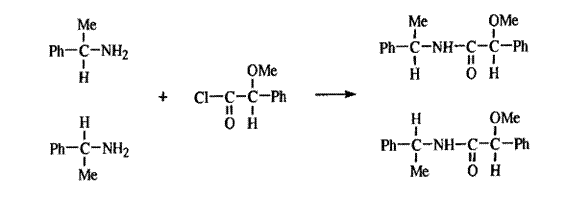
If we examined the NMR spectrum of the starting mixture, we would find only one peak (split into a doublet by the C—H) for the Me protons, since enantiomers give identical NMR spectra. But the two amides are not enantiomers and each Me gives its own doublet. From the intensity of the two peaks, the relative proportions of the two diastereomers, and hence of the original enantiomers, can be determined. Alternatively, the "unsplit" OMe peaks could have been used. This method was satisfactorily used to determine the optical purity of a sample of l-phenylethylamine (the case shown above), as well as other cases, but it is obvious that sometimes corresponding groups in diastereomeric molecules will give NMR signals that are too close together for resolution. In such cases, one may resort to the use of a different optically pure reagent. The 13C NMR can be used in a similar manner. It is also possible to use these spectra to determine the absolute configuration of the original enantiomers by comparing the spectra of the diastereomers with those of the original enantiomers. From a series of experiments with related compounds of known configurations it can be determined in which direction one or more of the 1H or 13C NMR peaks are shifted by formation of the diastereomer. It is then assumed that the peaks of the enantiomers of unknown configuration will be shifted the same way.
A closely related method does not require conversion of enantiomers to diastereomers but relies on the fact that (in principle, at least) enantiomers have different NMR spectra in a chiral solvent, or when mixed with a chiral molecule (in which case transient diastereomeric species may form). In such cases, the peaks may be separated enough to permit the proportions of enantiomers to be determined from their intensities. Another variation, which gives better results in many cases, is to use an achiral solvent but with the addition of a chiral lanthanide shift reagent such as tris[3-trifiuoroacetyl-J-camphorato]europium(III). Lanthanide shift reagents have the property of spreading NMR peaks of compounds with which they can form coordination compounds, for examples, alcohols, carbonyl compounds, amines, and so on. Chiral lanthanide shift reagents shift the peaks of the two enantiomers of many such compounds to different extents.
Another method, involving gas chromatography, is similar in principle to the NMR method. A mixture of enantiomers whose purity is to be determined is converted by means of an optically pure reagent into a mixture of two diastereomers. These diastereomers are then separated by gas chromatography (GC) and the ratios determined from the peak areas. Once again, the ratio of diastereomers is the same as that of the original enantiomers. High-pressure liquid chromatography (HPLC) has been used in a similar manner and has wider applicability. The direct separation of enantiomers by gas or liquid chromatography on a chiral column has also been used to determine optical purity.
Other methods involve isotopic dilution, kinetic resolution, 13C NMR relaxation rates of diastereomeric complexes, and circular polarization of luminescence.
4. Think of three reasons you liked the text and three reasons you didn’t like it. Share and compare your reasons with other students. Find out how many other students share your opinion.
5.Write down the skills required to master the material presented in the text. Talk about whether you have these skills and what kinds of experiences you have/should have that could help you to cope with the task.
Listening
1.Presentation: useful tips.
So what makes a good presentation? In this unit, we hear some tips from people who have made presentations about how to make yours more effective and enjoyable for your audience. Think about the presentations that you have been to. What were the characteristics of the good ones?

2. X Listento some advices from people who have made presentations and see if you can hear some of the tips above.
Дата добавления: 2015-10-28; просмотров: 129 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Render the text. | | | Look at Business English section and write an inquiry letter to the Supraveni Chemicals Private Limited (you can find information in the writing section of the unit 4). |