
Читайте также:
|
4. The text belowshows how the Cahn–Ingold–Prelog system, called the sequence rules, is used to specify the absolute configuration at the stereogenic center in (+)-2-butanol. Put the steps of the system into correct order.
Absolute configuration according to the Cahn–Ingold–Prelog Notational System
gives that the absolute configuration of (+)-2-butanol is
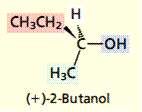
1. Orient the molecule so that the lowest ranked substituent points away from you.
2. If the order of decreasing precedence of the three highest ranked substituents appears in a clockwise sense, the absolute configuration is R (Latin rectus, “right,” “correct”). If the order of decreasing precedence is anticlockwise, the absolute configuration is S (Latin sinister, “left”).
3. Identify the substituents at the stereogenic center, and rank them in order of decreasing precedence according to the system described in Section 5.4. Precedence is determined by atomic number, working outward from the point of attachment at the stereogenic center.
4. Draw the three highest ranked substituents as they appear to you when the molecule is oriented so that the lowest ranked group points away from you.
Looking at the figure below explain the principles of the Fischer projection.

Speaking
1. Using conversational gambits given in the Appendix 2 construct a dialogue “Why do I like (dislike) stereochemistry”.
2. Match the following terms with their definition and give any additional information about them:
| 1 Stereoisomers | a) Stereoisomers that are not enantiomers | |
| 2 Enantiomers | b)One that rotate plane-polarized light are optically active (chiral). Compounds designated "l" rotate the plane of the polarized light counterclockwise. Compounds designated "d" rotate the plane of the polarized light clockwise | |
| 3 Diastereomers | c)An sp3carbon with four different substituents, often referred to as a chiral carbon. Stereoisomers are formed by changing the orientation of the substituents of the chiral carbon. | |
| 4 Asymmetric carbon | d)Isomers that have identical connectivity but different spatial configurations: two types are enantiomers and diastereomers. | |
| 5 Optically active compounds | e)If you can draw an internal mirror plane through a molecule (drawing the plane down the center of an atom is allowed), the molecule is not optically active and is called achiral. | |
| 6 General rule of thumb | f)Stereoisomers that are nonsuperimposable mirror images of each other | |
Дата добавления: 2015-10-28; просмотров: 144 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| The Scientific Method | | | Render the following text. |