
(Ursk, Kemerovo region, Russia)
Myagkaya I.N., Lazareva E.V., Zayakina S.B., Zhmodik S.M.
V.S. Sobolev Institute of Geology and Mineralogy SB RAS, Novosibirsk, Russia
i_myagkaya@mail.ru
The main focus of the reported study of tailing pits at an ore-processing plant (Ursk, Kemerovo region, Russia)has been on environmental geochemistry issues, namely the effect of chemicals on the environment and living organisms [1, 2]. However, tailings often store considerable amounts of precious components, including noble metals, which get into the solution while leaching, are transported away from the pits, and can re-precipitate at various geochemical barriers. Processing of metals with the use of organic matter has been explored since long ago, mostly with the examples of black shale and coal [3-5]. We investigated the speciation of gold in peat which is interacting with gold processing tailings.
The Ursk tailing pit, operated for more than 50 years, contains wastes from hardening of the Ursk ores, with the gold grade reaching 4 ppm. The processed waste primary ore and ore from the oxide zone are piled up in two 10-12 m piles. Being not fastened, the waste material has been washed out during floods and rainfalls for the whole period of operation. There is a natural creek flowing along the valley at the site, which becomes acid on draining the tailings. The acid waters have burnt out the swampy area downstream of the tailing pits, covered it with the shed material, and killed all vegetation as far as the Ur River (a tributary of the Inya). Leaching (oxidation), dissolution, and redeposition of elements from the acid waters, as well as transport of the waste material, have produced a halo around the pits. For the time the tailings exist, the shed material and the drainage waters have interacted with peat in the swampy area.
We analyzed peat sampled from tussocks and from 0.5 m below the ground surface, especially the peat that interacts with the wastes of both oxide gold zone and primary ore in the acid creek channel. The samples of tussocks and buried peat contain some amount of clastic wastes, mainly barite and quartz, or less often pyrite. There are also secondary phases, such as pseudo-cubic jarosite, concentric iron hydroxides (Fe III), faceted flat barite druses, framboidal pyrite, etc.).
The speciation of Au has been determined using the selective extraction technique. The following fractions have been identified: the water-soluble forms, these associated with organics/sulfides and with the Fe (III) hydroxides/oxides and residue (Fig. 1). The element abundances in aliquots were determined by ICP-MS. As the results showed, some gold is linked with water-soluble compounds. Aqueous fluids are known to carry gold in the thiosulfate form in the conditions of sulfide oxidation [6]. For organic-bearing substances, it is most probable that the soluble gold-bearing complexes are fulvic-acid organic-mineral varieties which are favorable for migration of the element [7]. About 1.5 ppm of gold is leached from peat at the next stage. Unfortunately, the available method does not allow discriminating between the gold related with organic matter and that associated with sulfides. The presence of water-soluble gold compounds suggests that some gold occurs in organic complexes, most likely with humic acids [8, 9]. A part of gold is found in secondary Fe(III) compounds.
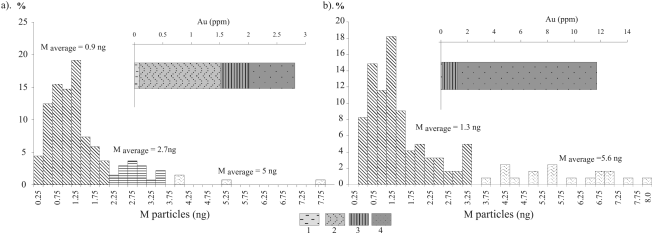
Fig. Weight histograms of Au contents in peat (a: tussock that interacts with layered wastes of two kinds; b: peat buried under oxide zone wastes) and gold species: 1- water-soluble; 2- associated with organic matter and/or sulfides, 3- associated with iron hydroxides; 4- non-decomposed residue.
The gold species in the peat that interacts simultaneously with the wastes of both primary ore and oxide zone are mostly those soluble in water and in organic complexes (Fig. 1 a). Gold related with iron compounds occurs near the surface at both sampling sites (0.3-2 ppm). Beneath the oxide zone wastes, along ephemeral streams, there are hard ferric crusts consisting of Fe(III) compounds forming at the boundary with tussocks. Up to 2 ppm Au are related with organic matter and/or sulfide material. High gold concentrations (up to 7.7-10.5 ppm) are due also to nuggets which make 70-80 % of the bulk. Gold contents range within 10.6-11.7 ppm in peat under the wastes of oxide zone and is from 2 to 7.5 ppm in that covered by both types of wastes; nugget gold is 1.3 ppm on the average in the samples from the surface and 0.6 ppm Au in the buried peat.
The sizes of gold particles were estimated using the Auger Electron Spectroscopy (AES). The gold particles from buried peat beneath the oxide zone ore wastes split into two groups (Fig. 1 B): finer particles (1.3 ng on the average) and coarser ones (5.6 ng). The tussock peat which interacts with waste material of both kinds contains gold particles of three size groups: 0.9, 2.7, and 5 ng (Fig. 1 a). The stepwise leaching data are in a good agreement with the AES results. We suggest the coarser particles to be the nugget form, the finer ones to be associated with sulfides (especially, pyrite), or with iron hydroxides, while the smallest particles may be bound in organic compounds.
Thus, gold redeposited in peat occurs in minor amounts in the form of water-soluble compounds, is partly associated with organics and/or sulfides, and some is the species related with iron hydroxides. The gold contents are the highest in places of nugget deposition.
The study was supported by RFBR 11-05-01020-а and IP of the SB RAS 94.
References:
1. Shcherbakova I.N., Gustaitis M.A., Lazareva E.V., Bogush A.A. Migration of heavy metals (Cu, Pb, Zn, Fe, Cd) in the halo of the Ursk’s tailing pit (Kemerovo region) // Chemistry for Sustainable Development. 18. 2010. No.5. p. 624-633. (Published in Russian).
2. Gustaitis M.A., Lazareva E.V., Bogush A.A., Shuvaeva O.V., Shcherbakova I.N., Polyakova E.V., Badmaeva Zh.O., Anoshin G.N. Distribution of mercury and its species in the zone of sulphide tailing // Doklady Earth Sciences. 2010. Vol. 432. part 2. p. 778-782. Russian version is Doklady Akademii Nauk. 2010. Vol. 432. No. 5. p. 655-659.
3. Baruah M.K., Kotoky P., Borah G.S. Gold in high sulphur Indian coals // Fuel, 77. 1998. p. 1867-1868.
4. Arbuzov S.I., Rikhvanov L.P., Maslov S.G., Arhipov V.S., Belyaeva A.M. Anomalous gold contents in brown coals and peat in the south-eastern region of the Western-Siberian platform // Int. J. Coal Geol. 2006. Vol. 68. No.3-4. р. 127-134.
5. Sazonov V.N, Koroteev V.A, Ogorodnikov V.N., Polenov J.A., Velikanov A.J. Gold in black shale of the Urals // Lithosphere. 2011. No.4. p. 70–92. (Published in Russian).
6. Greffie C., Benedetti M.F., Parron C., Amouric M. Gold and iron oxide associations under supergene conditions: an experimental approach // Geochim. Cosmochim. Acta. Vol. 60. 1996. p. 1531-1542.
7. Bowell R.J., Gize A.P., Foster R.P. The role of fulvic acid in the supergene migration of gold in tropical rain forest soils // Geochimica et Cosmochim. Acta. Vol. 57. 1993. p. 4179-4190.
8. Varshal G. M., Velyuhanova T. K., Chhetiya D.N., Cholin J. V., Tyutyunnik O.A., Koshcheeva I. J., Korochantsev A. V. Sorption on humic acids as the basis of primary accumulation mechanism of gold and platinum group elements in black shales // Lithology and Mineral Resources. 2000. 6. p. 605-613. (Published in Russian).
9. Kuimova N. G., Pavlova L. M., Sorokin A. P., Noskova L. P., Sergeyeva A. G. Experimental simulation of gold concentration in peat // Lithosphere, 2011, No. 4, p.131-136. (Published in Russian).
Дата добавления: 2015-10-29; просмотров: 126 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Laboratory experiments | | | Chemical composition of natural waters on the atomic power station building site |