
|
Читайте также: |
Fig. 6-8—Penetration of Zn parallel and perpendicular to the [011] rotation axis of Al
bicrystals. [I. Herbeuval, M. Biscondi, C. Goux, Mem. Sci. Rev. Met., 70 (1973) 39.]
Clearly there is a variation with angle and a minimum at the coherent twin orientation (/ = 3). The lack of symmetry reflects scatter in the data and scatter in the misorientation of the bicrystals used.
Temperature Dependence. Experimental results clearly indicate that the activation energy for grain boundary diffusion is less than that for lattice diffusion, while Do is about the same for the two. This is born out by the data in Table 6-1. The samples used to get this data were polycrystalline and the observed penetration will be weighted toward high angle grain boundaries with higher values of Db8.
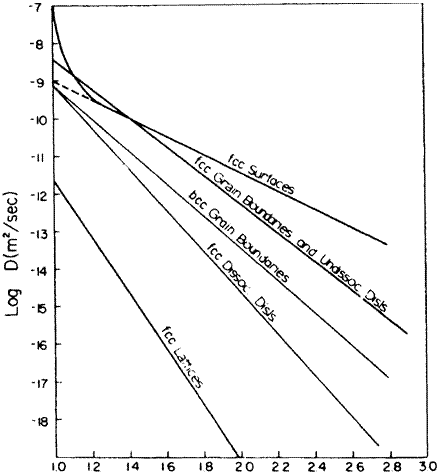
If one compares DO for different types of grain boundaries, the results can be rationalized in terms of the atomic packing in the given boundary compared to the packing (density) in a perfect lattice. If there is more open space a grain boundary, some of the atoms will be able to jump with a lower activation energy than if the atoms are more tightly packed. A lower Q will translate into a higher value of D. Such behavior is shown in Fig. 6-9 which represents the spectrum of data for D in: boundaries made of undissociated dislocations or high angle boundaries (Qb/Q, = 0.4-0.5), dissociated dislocations and twist boundaries (Qb/Qi = 0.6-0.8), and lattice diffusion. For the same misorientation between two grains, diffusion down a twist boundary is 10 to 100 times slower than down the tilt boundary. Examination of a tilt boundary shows that it has clear regions of low density while a twist boundary involving screw dislocations has little dilatation and primary shear strains. (The surface diffusion curve is discussed below.)







 Tm~T(K)
Tm~T(K)
Fig. 6-9—Diffusivity vs. reduced reciprocal temperature for various diffusion paths in metals. [N. Gjostein, in Diffusion, ASM, Metals Park, OH, (1973) p. 241-74.]
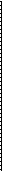 Mechanism. Calculations using molecular dynamics models have been made on models of dislocations, and a few on segments of grain boundaries. Fig. 6-10 shows several consecutive planes normal to the plane of a 6 = 36.9° [1001 tilt boundary (I = 5) in a bcc lattice. This boundary is 'high angle', but also has a relatively small repeat distance along the boundary in the planes shown. Thus the size of the model needed for calculations is reasonable. The results for diffusion in such a grain boundary indicate that both the motion energy and the formation energy of a vacancy are less than in the lattice, with the reduction in the energy of motion being about twice the reduction in the formation energy.1° Other conclusions are:
Mechanism. Calculations using molecular dynamics models have been made on models of dislocations, and a few on segments of grain boundaries. Fig. 6-10 shows several consecutive planes normal to the plane of a 6 = 36.9° [1001 tilt boundary (I = 5) in a bcc lattice. This boundary is 'high angle', but also has a relatively small repeat distance along the boundary in the planes shown. Thus the size of the model needed for calculations is reasonable. The results for diffusion in such a grain boundary indicate that both the motion energy and the formation energy of a vacancy are less than in the lattice, with the reduction in the energy of motion being about twice the reduction in the formation energy.1° Other conclusions are:
 • Vacancy jumps along the grain boundary core, of the sort B —p D —> B or B ---> C —> B are the most frequent.
• Vacancy jumps along the grain boundary core, of the sort B —p D —> B or B ---> C —> B are the most frequent.



 Fig. 6-10--Structure of IR = 5 [110] tilt boundary in bcc lattice showing repeated triangular units, and jump sequences along the core designated by arrows between atoms with letters beside them. (after R. W. Balluffi)
Fig. 6-10--Structure of IR = 5 [110] tilt boundary in bcc lattice showing repeated triangular units, and jump sequences along the core designated by arrows between atoms with letters beside them. (after R. W. Balluffi)
· Movement in the boundary normal to the core is much slower than along the core, that is, anisotropy in DO persists at the high angles.
n Interstitials can form relatively easily, but move slowly.
The interatomic potentials used for such calculations do not give accurate values of the energies involved, but authors working with models of a variety of metals, for both dislocations and grain boundaries, all agree that diffusion is primarily by vacancy motion. This conclusion is further strengthened by the observation that the activation volume for diffusion in high-angle grain boundaries in silver is larger for boundary diffusion than for lattice diffusion, namely Vb is 1.1 f2 while Vi = 0.9 fl." This large Vb also lends credence to the basic idea that vacancies exist in the boundary which are very similar to those existing in the lattice.
Alloying Effects. Alloying elements often segregate at grain boundaries or dislocations. This is especially true for elements whose solubility is low. This can have two distinctly different effects:
 HG. Martin, D. A. Blackburn, Y. Adda, Phys. Status Solidi, 23 (1967) 223.
HG. Martin, D. A. Blackburn, Y. Adda, Phys. Status Solidi, 23 (1967) 223.
· 
 The binding to the high diffusivity path raises the mean diffusion coefficient of the impurity because it spends a larger fraction of the time where it diffuses faster.
The binding to the high diffusivity path raises the mean diffusion coefficient of the impurity because it spends a larger fraction of the time where it diffuses faster.
· The solute can change the boundary structure, for example fill in open spaces and make it harder for atoms to move along the boundary or dislocation.
The first effect of segregation can most easily be treated by considering the boundary condition between the grain boundary and the lattice. Rather than the concentration being equal in the two regions, Eq. (6-5) becomes
cb(5/2) = Kc1(3/2) (6-11)
Subsequent equations are unchanged except that DO is replaced in all equations by KDb8.
The second effect cannot be quantified as simply, nor is there any widely accepted theory of solute diffusion or the effect of solutes on solvent diffusion, as there is for lattice diffusion.
6.3 DISLOCATION & GRAIN SIZE EFFECTS
The treatment above dealt with the measurement of Db and Di, in bicrystals. Consider now the effect of randomly oriented dislocations, or a fine grain size. These are of interest in studying the kinetics of diffusion limited processes at or below half the melting temperature, and are central in determining the rate of diffusion in fine grained thin films.
There is great similarity between the results for diffusion enhanced by a three dimensional array of dislocationsu and that arising from a 3-D array of grain boundaries.13 We will describe the results for dislocations, and then compare the results with those for boundaries. Consider the regular array of dislocation pipes of radius 'a' and separation 2Z as shown schematically in Fig. 6-11. They are normal to the free surface, which has solute on it. The section can show three different types of solute distributions depending on the ratio of the mean diffusion distance in the lattice \//sit- to the separation Z, and remembering that Di, is always much larger than DI.
· 1/D1t Z, an atom interacts with several dislocations in diffusing
this far through the lattice. The dislocations increase the effective
'2A. D. LeClaire, A. Rabinovitch, in Diffusion in Crystalline Solids, ed. G. Murch, A. Nowick, Academic Press (1984) pp. 259-319.
'D. Gupta, D. R. Campbell, P. S. Ho, in Thin Films—Interdiffusion and Reactions, eds. Poate, Tu & Mayer, J. Wiley & Sons, (1978) pp. 161-242.
















 Fig. 6-11—Schematic representation of the various concentration profiles that can de-
Fig. 6-11—Schematic representation of the various concentration profiles that can de-
velop with different ratios of V(Dit)/Z. The solute source is at the top of each figure.
diffusivity Deff for the solid, so that the penetration depth is greater than it would be without dislocations. The advancing isoconcentration lines are relatively flat near the dislocations. This will be re ferred to as 'A-kinetics.'
· VD it C Z, but VDit a. The solute field around each dislocation develops independently of its neighbors, and the situation is similar to the case of grain boundary diffusion in bicrystals analyzed above. B-kinetics.
· VD1t < a, Diffusion occurs only in the dislocation pipes with no loss to the surrounding lattice. This is rare in bulk samples, but in a thin film at T =0.5 T„„ 131,/D1 can equal 108 (see estimate for Ag above) and diffusion through a one micron thick film can occur without any solute being lost to the lattice. C-kinetics.
Дата добавления: 2015-10-29; просмотров: 114 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Several predictions stem from this model. | | | These three cases are next treated in more detail. |