
Читайте также:
|
Molecular Biochemistry II
http://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb2/part1/fatcatab.htm
Contents of this page:
Fatty acids & triacylglycerols
Fatty acid activation & transport
Fatty acid b-Oxidation
Ketone bodies
| A 16-carbon fatty acid, with numbering conventions, is shown at right. Most naturally occurring fatty acids have an even number of carbon atoms. The pathway for catabolism of fatty acids is referred to as the b-Oxidation Pathway, because oxidation occurs at the b-carbon (C3). | 
|
| Triacylglycerols (triglycerides) are the most abundant dietary lipids. They are the form in which we store reduced carbon for energy. Each triacylglycerol has a glycerol backbone to which are esterified 3 fatty acids. Most triacylglycerols are "mixed." The three fatty acids differ in chain length and number of double bonds Lipid digestion, absorption and transport will be covered separately. Lipases hydrolyze triacylglycerols, releasing one fatty acid at a time, producing diacylglycerols,and eventually glycerol.. | 
|
| Glycerol arising from hydrolysis of triacylglycerols is converted to the Glycolysis intermediate dihydroxyacetone phosphate, by reactions catalyzed by: (1) Glycerol Kinase (2) Glycerol Phosphate Dehydrogenase. | 
|
Free fatty acids, which in solution have detergent properties, are transported in the blood bound to albumin, a serum protein produced by the liver.
Several proteins have been identified that facilitate transport of long chain fatty acids into cells, including the plasma membrane protein CD36.
| Fatty acid activation: Fatty acids must be esterified to Coenzyme Abefore they can undergo oxidative degradation, be utilized for synthesis of complex lipids (e.g., triacylglycerols or membrane lipids), or be attached to proteins as lipid anchors. Acyl-CoA Synthases (Thiokinases), associated with endoplasmic reticulum membranes and the outer mitochondrial membrane, catalyze activation of long chain fatty acids, esterifying them to coenzyme A, as shown at right. This process is ATP-dependent, and occurs in 2 steps. There are different Acyl-CoA Synthases for fatty acids of different chain lengths. Exergonic hydrolysis of PPi (P~P), catalyzed by Pyrophosphatase, makes the coupled reaction spontaneous. Overall, two ~P bonds of ATP are cleaved during fatty acid activation. The acyl-coenzyme A product includes one "high energy" thioester linkage. | 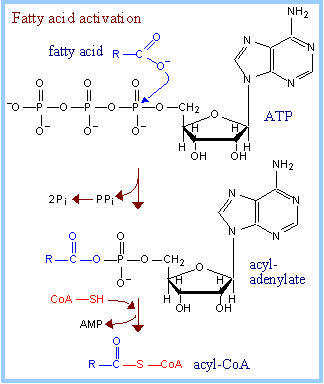
|
Summary of fatty acid activation:
Overall: fatty acid + ATP + HS-CoA à acyl-CoA + AMP + 2 Pi
| Fatty acids are degraded in the mitochondrial matrix via the b-Oxidation Pathway. For most steps of the pathway there are multiple enzymes specific for particular fatty acid chain lengths. Many of the constituent enzymes are soluble proteins located in the mitochondrial matrix. But enzymes specific for very long chain fatty acids are associated with the inner mitochondrial membrane, facing the matrix. Fatty acyl-CoA formed outside the mitochondria can pass through the outer mitochondrial membrane, which contains large VDAC channels, but cannot penetrate the mitochondrial inner membrane. | 
|
| Transfer of the fatty acid moiety across the mitochondrial inner membrane involves carnitine. Carnitine Palmitoyl Transferases catalyze transfer of a fatty acid between the thiol of Coenzyme A and the hydroxyl on carnitine. Carnitine-mediated transfer of the fatty acyl moiety into the mitochondrial matrix is a 3-step process, as presented below. | 
|
| 1.Carnitine Palmitoyl Transferase I, an enzyme associated with the cytosolic surface of the outer mitochondrial membrane, catalyzes transfer of a fatty acid from ester linkage with the thiol of coenzyme A to the hydroxyl on carnitine. 2. Carnitine Acyltransferase, an antiporter in the inner mitochondrial membrane, mediates transmembrane exchange of fatty acyl-carnitine for carnitine. 3. Within the mitochondrial matrix (or associated with the matrix surface of the inner mitochondrial membrane, Carnitine Palmitoyl Transferase II catalyzes transfer of the fatty acid from carnitine to coenzyme A. (Carnitine exits the matrix in step 2.) The fatty acid is now esterified to coenzyme A within the mitochondrial matrix. | 
|
Control of fatty acid oxidation is exerted mainly at the step of fatty acid entry into mitochondria.
| Malonyl-CoA (which is also a precursor for fatty acid synthesis) inhibits Carnitine Palmitoyl Transferase I. Malonyl-CoA is produced from acetyl-CoA by the enzyme Acetyl-CoA Carboxylase. AMP-Activated Kinase a sensor of cellular energy levels, is allosterically activated by AMP, which is relatively high in concentration when [ATP] is low. Acetyl-CoA Carboxylase is inhibited when phosphorylated by AMP-Activated Kinase, leading to decreased production of malonyl-CoA. The decrease in malonyl-CoA concentration leads to increasedactivity of Carnitine Palmitoyl Transferase I. The resulting increased fatty acid oxidation generates acetyl-CoA, for entry into Krebs cycle with associated ATP production. | 
|
AMP-Activated Kinase functions under a variety of conditions that lead to depletion of cellular ATP (reflected as increased AMP), including glucose deprivation, exercise, hypoxia and ischaemia.
b-Oxidation Pathway:
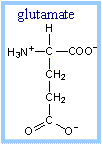
| Step 1. Acyl-CoA Dehydrogenase catalyzes oxidation of the fatty acid moiety of acyl-CoA, to produce a double bond between carbon atoms 2 and 3. There are different Acyl-CoA Dehydrogenases for short (4-6 C), medium (6-10 C), long and very long (12-18 C) chain fatty acids. Very Long Chain Acyl-CoA Dehydrogenase is bound to the inner mitochondrial membrane. The others are soluble enzymes located in the mitochondrial matrix. FAD (below) is the prosthetic group that functions as electron acceptor for Acyl-CoA Dehydrogenase. Proposed mechanism: A glutamate side-chain carboxyl extracts a proton from the a-carbon of the substrate, facilitating transfer of 2 e- with H+ (a hydride) from the b position to FAD. The reduced FAD accepts a second H+, yielding FADH2. The carbonyl oxygen of the thioester substrate is hydrogen bonded to the 2'-OH of the ribityl moiety of FAD, giving this part of FAD a role in positioning the substrate and increasing acidity of the substrate a-proton. The reactive glutamate and FAD are on opposite sides of the substrate at the active site. Thus the reaction is stereospecific, yielding a trans double bond in enoyl-CoA. | 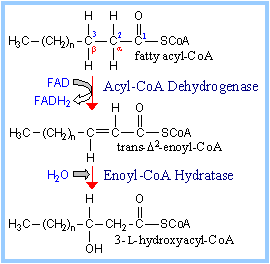 Steps 1 & 2 of b-Oxidation Pathway Steps 1 & 2 of b-Oxidation Pathway
|
| FADH2of Acyl CoA Dehydrogenase is reoxidized by transfer of 2 electrons to an Electron Transfer Flavoprotein (ETF), which in turn passes the electrons to coenzyme Q of the respiratory chain. | 
|
 Explore at right an example of an Acyl CoA Dehydrogenase (MCAD).
Explore at right an example of an Acyl CoA Dehydrogenase (MCAD).
|  MCAD MCAD
|
Step 2. Enoyl-CoA Hydratase catalyzes stereospecific hydration of the trans double bond produced in the 1st step of the pathway, yielding L-hydroxyacyl-Coenzyme A (diagram above right).
Step 3. Hydroxyacyl-CoA Dehydrogenasecatalyzes oxidation of the hydroxyl in the b position (C3) to a ketone. NAD+ is the electron acceptor.
 Step 4. b-Ketothiolase (b-Ketoacyl-CoA Thiolase) catalyzes thiolytic cleavage.
Proposed mechanism (see p. 919): A cysteine S attacks the b-keto C. Acetyl-CoA is released, leaving the fatty acyl moiety in thioester linkage to the cysteine thiol. The thiol of HSCoA displaces the cysteine thiol, yielding fatty acyl-CoA (2 C shorter).
A membrane-bound trifunctional protein complex with two subunit types expresses the enzyme activities for steps 2-4 of the b-oxidation pathway for long chain fatty acids. Equivalent enzymes for medium and short chain length fatty acids are soluble proteins of the mitochondrial matrix.
Step 4. b-Ketothiolase (b-Ketoacyl-CoA Thiolase) catalyzes thiolytic cleavage.
Proposed mechanism (see p. 919): A cysteine S attacks the b-keto C. Acetyl-CoA is released, leaving the fatty acyl moiety in thioester linkage to the cysteine thiol. The thiol of HSCoA displaces the cysteine thiol, yielding fatty acyl-CoA (2 C shorter).
A membrane-bound trifunctional protein complex with two subunit types expresses the enzyme activities for steps 2-4 of the b-oxidation pathway for long chain fatty acids. Equivalent enzymes for medium and short chain length fatty acids are soluble proteins of the mitochondrial matrix.
|  Steps 3 & 4 of b-Oxidation Pathway Steps 3 & 4 of b-Oxidation Pathway
|
Summary of one round of the b-oxidation pathway:
fatty acyl-CoA + FAD + NAD+ + HS-CoA à
fatty acyl-CoA (2 C shorter) + FADH2 + NADH + H+ + acetyl-CoA
The b-oxidation pathway is cyclic. The product, 2 carbons shorter, is the input to another round of the pathway. If, as is usually the case, the fatty acid contains an even number of C atoms, in the final reaction cycle butyryl-CoA is converted to 2 copies of acetyl-CoA.
ATP production:
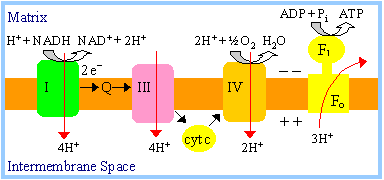
| 
|
Fatty acid oxidation is a major source of cellular ATP (see problem in today's studio exercise).
Human genetic diseases have been identified that involve mutations in the plasma membrane fatty acid transporter CD36; Carnitine Palmitoyltransferases I and II (required for transfer of fatty acids into mitochondria); Acyl-CoA Dehydrogenases for various chain lengths of fatty acids; Hydroxyacyl-CoA Dehydrogenases for medium and short chain length fatty acids; Medium Chain b-Ketothiolase, the trifunctional protein complex; and Electron Transfer Flavoprotein (ETF).
Symptoms vary depending on the specific genetic defect but may include hypoglycemia and failure to increase ketone body production during fasting, fatty degeneration of the liver; heart and/or skeletal muscle defects, maternal complications of pregnancy, and sudden infant death (SIDS). Hereditary deficiency of Medium Chain Acyl-CoA Dehydrogenase (MCAD), the most common genetic disease relating to fatty acid catabolism, has been linked to SIDS.
The reactions presented above accomplish catabolism of a fatty acid with an even number of carbon atoms and no double bonds. Additional enzymes deal with catabolism of fatty acids with an odd number of carbon atoms or including double bonds.
| b-Oxidation of very long chain fatty acids also occurs within peroxisomes. FAD is electron acceptor for peroxisomal Acyl-CoA Oxidase, which catalyzes the first oxidative step of the pathway. The resulting FADH2 is reoxidized in the peroxisome producing hydrogen peroxide: FADH2 + O2à FAD + H2O2 The peroxisomal enzyme Catalase degrades H2O2 by the reaction: 2 H2O2à 2 H2O + O2 These reactions produce no ATP. Once fatty acids are reduced in length within the peroxisomes they may shift to the mitochondria to be catabolized all the way to CO2. Carnitine is involved in transfer of fatty acids into and out of peroxisomes. Serious genetic diseases are associated with defects in or deficiency of enzymes of the peroxisomal b-oxidation system. Peroxisomes also contain enzymes for an essential a-oxidation pathway that degrades fatty acids having methyl branches, such as phytanic acid, a breakdown product of chlorophyll. | 
|
| Ketone bodies: During fasting or carbohydrate starvation, oxaloacetate is depleted in liver because it is used for gluconeogenesis. This impedes entry of acetyl-CoA into Krebs cycle. Acetyl-CoA then is converted in liver mitochondria to ketone bodies, acetoacetate and b-hydroxybutyrate. | 
|
| Three enzymes are involved in synthesis of ketone bodies: b-Ketothiolase. The final step of the b-oxidation pathway runs backwards, condensing 2 acetyl-CoA to produce acetoacetyl-CoA, with release of one CoA. HMG-CoA Synthase catalyzes condensation of a third acetate moiety (from acetyl-CoA) with acetoacetyl-CoA to form hydroxymethylglutaryl-CoA (HMG-CoA). HMG-CoA Lyase cleaves HMG-CoA to yield acetoacetate plus acetyl-CoA. | 
|
| b-Hydroxybutyrate Dehydrogenase catalyzes inter-conversion of the ketone bodies acetoacetate and b-hydroxybutyrate. Ketone bodies are transported in the blood to other cells, where they are converted back to acetyl-CoA (diagram p. 929) for catabolism in Krebs cycle, to generate ATP. While ketone bodies thus function as an alternative fuel, amino acids must be degraded to supply input to gluconeogenesis when hypoglycemia occurs, since acetate cannot be converted to glucose. (See sections on gluconeogenesis and amino acid catabolism.) | 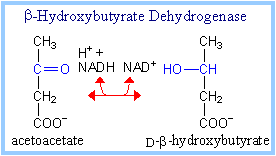
|
Дата добавления: 2015-10-29; просмотров: 154 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Expérience 3. La digestion des graisses. L'influence des acides biliaires sur l’activité de la lipase pancréatique. | | | Поддержания целостности данных, т.е. сохранения существующих межтабличных связей при вводе, изменении и удалении записей. |