
Читайте также:
|
Research question:
Experiment to investigate the enthalpy change in kJ/mol of combustion of 5 different types of 40% alcohol beverages: Red Label Johnnie Walker Whiskey, Richelieu Cognac, Cointreau liqueur, Molinari Sambuca extra, Tequila Sierra Reposado. 13 ml of each beverage will be burnt at a distance of 3.0 cm between the bottom of the copper cup filled with 200 ml of water and the crucible. Water will be measured with the help of the 250 ml measuring cylinder ( 1.5 ml). Initial and final temperatures will be measured with a Digital Temperature Probe (
1.5 ml). Initial and final temperatures will be measured with a Digital Temperature Probe ( 0.1
0.1  C) in order to determine the enthalpy change for each type of alcohol. Five trials for each beverage will be taken.
C) in order to determine the enthalpy change for each type of alcohol. Five trials for each beverage will be taken.
Hypothesis:
I predict that all alcohol beverages will have different heat capacity's and enthalpy changes, because of their's different contents. To my mind some components of the compounds are more flammable, combustible, and can burn more and therefore, the difference in temperature will increase together with an enthalpy change.
Reason:
This experiment is a combustion investigation and all its theory is based on a combustion of the alcohol beverages. Combustion is an exothermic reaction, the heat will be given to the surroundings. Exothermic reactions have to take more energy to break chemical bonds than they need to form bonds. All alcohol beverages contain ethanol (C2H5-OH), which is very flammable. All chosen beverages have 40% of it.
Compete combustion of ethanol forms carbon dioxode and water vapor:
C2H5-OH (l) + 3O2 (g) → 2CO2 (g) + 3H2O (g)

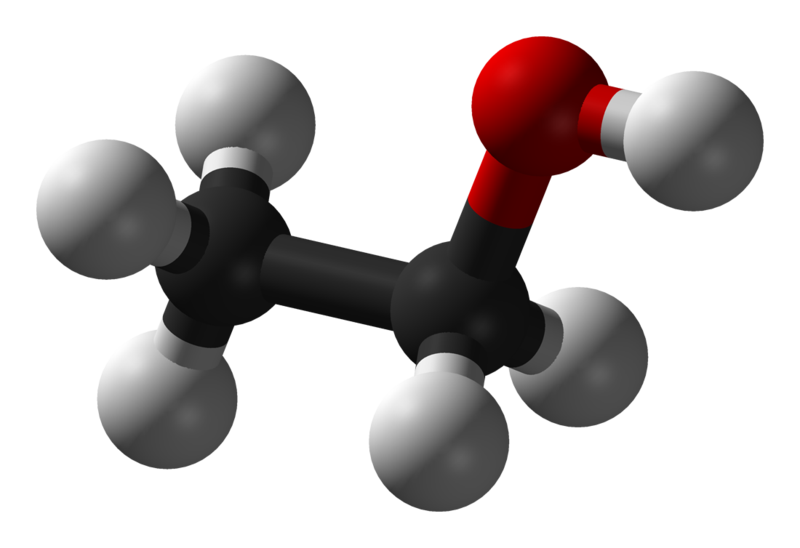
Diagram 1 Diagram 2
Diagram 1 and 2: showing the full structural formula and a ball-and-stick model
Variables:
Independent variables:
Five different 40% alcohol beverages and brands:
· Red Label Johnnie Walker Whiskey
· Richelieu Cognac
· Cointreau liqueur
· Molinari Sambuca extra
· Tequila Sierra Reposado
Dependent variables:
· Temperature change will be calculated by subtracting the initial temperature from the final temperature.
· Five different enthalpy changes will be calculated by using the formulas including the specific heat capacity of water (4.184 kJ/kgK), temperature change (±0.1  C) and mas of water (200 g,
C) and mas of water (200 g,  1.5 mL)
1.5 mL)
Controlled:
1. Amount of alcohol beverage will be always the same – 13 ml, measured with 25 ml measuring cylinder ( 0.38 ml).
0.38 ml).
2. Amount of water heated will be always the same – 200 ml, measured with 250 ml measuring cylinder ( 1.5 ml).
1.5 ml).
3. Distance between the bottom of the copper cup and the ceramic crucible with burning alcohol beverage will be always the same – 3 cm, measured with a ruler ( 0.1 mm).
0.1 mm).
4. The percentage of ethanol in all alcohol beverages will be the same – 40%.
Apparatus/ Materials:
· Ceramic Crucible
· Safety Goggles
· Clay Triangle
· Clamp stand
· Ring Stand
· Ruler ( 0.1 mm)
0.1 mm)
· 5 different types of alcohol beverages (40%): Red Label Johnnie Walker Whiskey
Richelieu Cognac
Cointreau liqueur
Molinari Sambuca extra
Tequila Sierra Reposado
· 25 ml Measuring cylinder ( 0.38 ml)
0.38 ml)
· 250 ml Measuring cylinder ( 1.5 ml)
1.5 ml)
· Stopwatch ( 0.1 s)
0.1 s)
· Water
· 500 ml Copper cup
· Texas TI-84 Plus Calculator
· Vennier Data Logger
· Digital Temperature Probe ( 0.1
0.1  C)
C)
· Matches
· Bunsen Burner
· Tongs
· 3 pipettes





ФОТКИ БЛЯТЬ
МАТЕРИАЛОВ.
ПОДПИШИ ИХ!
Set up the Equipment:
Prepare and set up all the apparatus needed for the practical:
1. The tripod was set up with a clay triangle, so the copper cup with 200 ml of water would safely stand during the experiments.
2. The ring stand was connected to the tripod underneath the copper cup.
3. The crucible was placed in the ring stand.
4. The length was measured with a ruler ( 0.1 mm) between the copper cup and the crucible in the ring stand.
0.1 mm) between the copper cup and the crucible in the ring stand.
5. Two measuring cylinders were prepared: 250 ml ( 1.5 ml) and 25 ml (
1.5 ml) and 25 ml ( 0.38 ml) with 3 pipettes.
0.38 ml) with 3 pipettes.
6. The Vennier Data Logger connected to Texas TI-84 Plus Calculator and Digital Temperature Probe was prepared. The Easy Data application was switched on.
Дата добавления: 2015-10-28; просмотров: 155 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| MUSTER / SAMPLE Antrag auf Erteilung / Verlängerung des Aufenthaltstitels | | | Фоточки запили чтоле |