
Method:
1. Two hundred ml of water was measured with a 250 ml measuring cylinder ( 1.5 ml) and poured into the copper cup.
1.5 ml) and poured into the copper cup.
2. The copper cup with water inside was placed on a clay triangle, on the clamp stand. The temperature of the water was taken with a Digital Temperature Probe ( 0.1
0.1  C).
C).
3. Thirteen ml of the first alcohol beverage was measured with a 25 ml measuring cylinder ( 0.38 ml) and pipettes. Then it was poured into the crucible.
0.38 ml) and pipettes. Then it was poured into the crucible.
4. The crucible was placed in the ring stand.
5. The Bunsen burner was lit up with matches.
6. The crucible with alcohol beverage was heated for approximately 15-20 seconds, until the alcohol was lighted up. Фоточку чтоле
7. After the alcohol started to burn, the clamp stand with a clay triangle and a copper cup with water inside was moved to the crucible.
8. The final temperature of the water was measured with the help of a Digital Temperature Probe ( 0.1
0.1  C) after the acohol was burnt completely. The Probe was placed in the middle of the copper cup for about 1 minute and was not touched by the bottom of the copper cup.
C) after the acohol was burnt completely. The Probe was placed in the middle of the copper cup for about 1 minute and was not touched by the bottom of the copper cup.
9. The gotten results were written in the table.
10. The experiment was repeated 4 more times with the same type of alcohol beverage in order to collect the relevant and sufficient data.
11. The steps 1-9 were repeated with the other types of alcohols.
12. The data taken was put in the results tables.
13. It was assumed, that 1 ml of alcohol = 1 g of alcohol, and 1 ml of water = 1 g of water.
14. The enthalpy change was calculated using the formula:
Heat energy (∆H) = mass (m) × specific heat capacity of water (c) × temperature change (∆T)
∆H = m × c × ∆T
14. The received and calculated data was analysed.
Calculations:
Specific Heat Capacity of Water = 4.184 J/g  C
C
Mass of Water = 200 g
Heat energy (∆H) = mass (m) × specific heat capacity of water (c) × temperature change (∆T)
∆H = m × c × ∆T
Enthalpy change for Molinari Sambuca extra:
Trial 1:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 24.2
C × 24.2  C = 20250.6 J/mol = 20.3 kJ/mol
C = 20250.6 J/mol = 20.3 kJ/mol
Trial 2:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 26.4
C × 26.4  C = 22091.5 J/mol = 22.1 kJ/mol
C = 22091.5 J/mol = 22.1 kJ/mol
Trial 3:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 32.8
C × 32.8  C = 27447.0 J/mol = 27.4 kJ/mol
C = 27447.0 J/mol = 27.4 kJ/mol
Trial 4:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 27.0
C × 27.0  C = 22593.6 J/mol = 22.6 kJ/mol
C = 22593.6 J/mol = 22.6 kJ/mol
Trial 5:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 33.1
C × 33.1  C = 27698.1 J/mol = 27.7 kJ/mol
C = 27698.1 J/mol = 27.7 kJ/mol
Enthalpy change for Tequila Sierra Reposado:
Trial 1:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 19.5
C × 19.5  C = 16317.6 J/mol = 16.3 kJ/mol
C = 16317.6 J/mol = 16.3 kJ/mol
Trial 2:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 20.8
C × 20.8  C = 17405.4 J/mol = 17.4 kJ/mol
C = 17405.4 J/mol = 17.4 kJ/mol
Trial 3:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 18.7
C × 18.7  C = 15648.2 J/mol = 15.6 kJ/mol
C = 15648.2 J/mol = 15.6 kJ/mol
Trial 4:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 20.7
C × 20.7  C = 17321.8 J/mol = 17.3 kJ/mol
C = 17321.8 J/mol = 17.3 kJ/mol
Trial 5:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 23.7
C × 23.7  C = 19832.2 J/mol = 19.8 kJ/mol
C = 19832.2 J/mol = 19.8 kJ/mol
Enthalpy change for Cointreau liqueur:
Trial 1:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 29.5
C × 29.5  C = 24685.6 J/mol = 24.7 kJ/mol
C = 24685.6 J/mol = 24.7 kJ/mol
Trial 2:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 32.6
C × 32.6  C = 27279.7 J/mol = 27.3 kJ/mol
C = 27279.7 J/mol = 27.3 kJ/mol
Trial 3:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 28.6
C × 28.6  C = 23932.5 J/mol = 23.9 kJ/mol
C = 23932.5 J/mol = 23.9 kJ/mol
Trial 4:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 25.1
C × 25.1  C = 21003.7 J/mol = 21.0 kJ/mol
C = 21003.7 J/mol = 21.0 kJ/mol
Trial 5:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 30.0
C × 30.0  C = 25104.0 J/mol = 25.1 kJ/mol
C = 25104.0 J/mol = 25.1 kJ/mol
Enthalpy change for Richelieu Cognac:
Trial 1:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 20.7
C × 20.7  C = 17321.8 J/mol = 17.3 kJ/mol
C = 17321.8 J/mol = 17.3 kJ/mol
Trial 2:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 21.8
C × 21.8  C = 18242.2 J/mol = 18.2 kJ/mol
C = 18242.2 J/mol = 18.2 kJ/mol
Trial 3:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 23.9
C × 23.9  C = 19999.5 J/mol = 20.0 kJ/mol
C = 19999.5 J/mol = 20.0 kJ/mol
Trial 4:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 24.3
C × 24.3  C = 20334.2 J/mol = 20.3 kJ/mol
C = 20334.2 J/mol = 20.3 kJ/mol
Trial 5:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 22.7
C × 22.7  C = 18995.4 J/mol = 19.0 kJ/mol
C = 18995.4 J/mol = 19.0 kJ/mol
Enthalpy change for Red Label Johnnie Walker Whiskey:
Trial 1:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 18.2
C × 18.2  C = 15229.8 J/mol = 15.2 kJ/mol
C = 15229.8 J/mol = 15.2 kJ/mol
Trial 2:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 16.9
C × 16.9  C = 14141.9 J/mol = 14.1 kJ/mol
C = 14141.9 J/mol = 14.1 kJ/mol
Trial 3:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 19.3
C × 19.3  C = 16150.2 J/mol = 16.2 kJ/mol
C = 16150.2 J/mol = 16.2 kJ/mol
Trial 4:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 20.4
C × 20.4  C = 17070.7 J/mol = 17.1 kJ/mol
C = 17070.7 J/mol = 17.1 kJ/mol
Trial 5:
∆H = m × c × ∆T = 200 g × 4.184 J/g  C × 18.3
C × 18.3  C = 15313.4 J/mol = 15.3 kJ/mol
C = 15313.4 J/mol = 15.3 kJ/mol
Error Calculations:
Ruler error
Minimum error =  × 100 % = 0.3 %
× 100 % = 0.3 %
Maximum error =  × 100 % = 0.3 %
× 100 % = 0.3 %
250 ml Measuring Cylinder error
Minimum error =  × 100 % = 0.74 %
× 100 % = 0.74 %
Maximum error =  × 100 % = 0.76 %
× 100 % = 0.76 %
Digital Temperature Probe error
Minimum error = 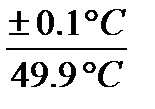 × 100 % = 0.2 %
× 100 % = 0.2 %
Maximum error =  × 100 % = 0.3 %
× 100 % = 0.3 %
Propagated error
Minimum error = 0.3% + 0.74% + 0.2% = 1.2%
Maximum error = 0.3% + 0.76% + 0.3% = 1.4%
Дата добавления: 2015-10-28; просмотров: 83 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Investigating the Enthalpy Change of different types of alcohol beverages | | | Observations/Qualitative data: напиши шо все по менискусу читала, всё по хардкору |