
Читайте также:
|
Graph 1: showing the enthalpy changes for 5 trials and the average enthalpy change for Molinari Sambuca extra.

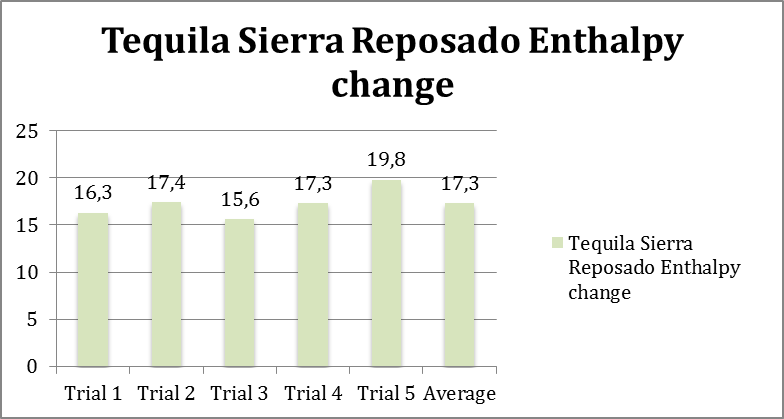
Graph 2: showing the enthalpy changes for 5 trials and the average enthalpy change for Tequila Sierra Reposado.
Graph 3: showing the enthalpy changes for 5 trials and the average enthalpy change for Cointreau liqueur.

Graph 4: showing the enthalpy changes for 5 trials and the average enthalpy change for Richelieu Cognac.

Graph 5: showing the enthalpy changes for 5 trials and the average enthalpy change for Red Label Johnnie Walker Whiskey.

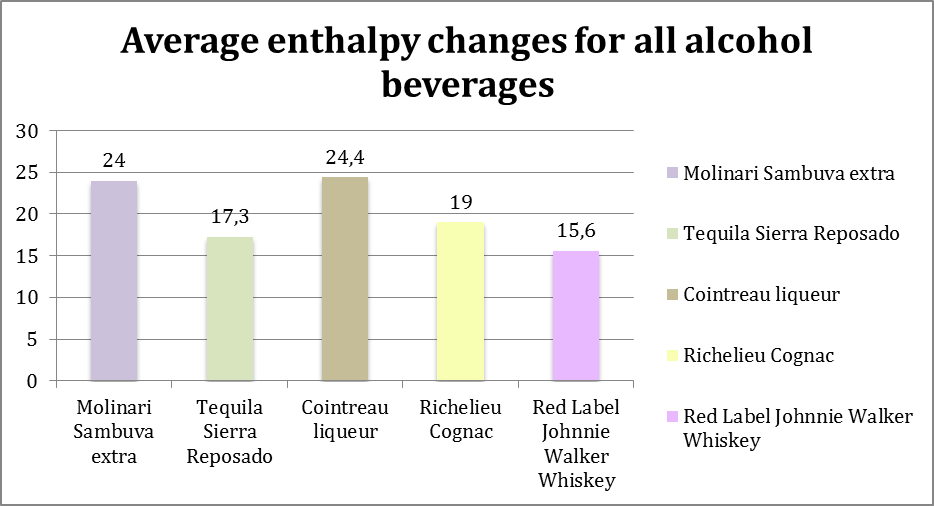
Graph 6: showing the average enthalpy changes for 5 different types of alcohol beverages (Molinari Sambuca extra, Tequila Sierra Reposado, Cointreau liqueur, Richelieu Cognac, and Red Label Johnnie Walker Whiskey) collected and calculated from 5 trials of each.
Conclusion and Evaluation:
Weaknesses and limitations:
· There was some lost of the heat, energy to the surroundings, because the system was open. If the system were closed, the results would be more precise because all of the energy would have gone directly to the copper cup, and then to the water.
· The 250 ml measuring cylinder had an uncertainty of ( 1.5 ml), and that affected the volume of water used, and made some influence upon the gotten resuls. Because the temperature depended on the amount of water used, and therefore it affected the calculated enthalpy change.
1.5 ml), and that affected the volume of water used, and made some influence upon the gotten resuls. Because the temperature depended on the amount of water used, and therefore it affected the calculated enthalpy change.
· The 25 ml measuring cylinder had an uncertainty of ( 0.38 ml), and that affected the volume of a alcohol beverage used to burn, which leads to an effect upon the calculated enthalpy change.
0.38 ml), and that affected the volume of a alcohol beverage used to burn, which leads to an effect upon the calculated enthalpy change.
· The Temperature Probe was pretty inaccurate with an uncertainty of ( 0.1 C
0.1 C  ). Also, it took some time for it to record the temperature, therefore the maximum temperature is very unprecised. This affected the calculated enthalpy change.
). Also, it took some time for it to record the temperature, therefore the maximum temperature is very unprecised. This affected the calculated enthalpy change.
· Some alcohol beverages (Red Label Johnnie Walker Whiskey, Richelieu Cognac, and Tequila Sierra Reposado) needed to be heated before burning. And they always lighted up very suddenly. It caused some loss of the heat to the surroundings, when the copper cup with water inside, on a clamp stand was on its way to the crucible. Also, sometimes the alcohol stopped to burn, but the combustion was not complete. So, some experiments had to be repeated more than 5 times.
· Some heat was absorbed by the ring clay triangle and the clamp stand.
· Sometimes, the alcohol was heated too much and it boiled, creating some discomfort and a need to repeat the trial from the beginning.
Improvements:
· Even though a copper cup was much better to use then a glass beaker, the experiment still could be improved by using a bomb calorimeter instead of a copper cup. Bomb calorimeter would converse more heat. Picture (1)

Picture 1: showing a bomb calorimeter
· More accurate equipment could be used in order to minimize the errors: 200 ml and 15 ml measuring pipettes with uncertainties of (± 0.1ml).
· More accurate equipment (with 2 decimal places) for the temperature measurements could be used. Picture 2

Picture 2: showing better equipment (with 2 decimal places) for the temperature measurements
· A tripod could be used for holding the copper cup, in order to escape the absorption of heat by the clay triangle and the clamp stand.
· Alcohol with a higher % of ethanol could be used in order to simplify the experiment and make it easy to light up the alcohol drink.
Bibliography:
· http://www.liker.info/archives/82
· http://www.21food.com/products/tequila-sierra-reposado-gold-288829.html
· http://dominicanewsonline.com/news/all-news/court/father-of-2-loses-job-over-red-label-whiskey/
· http://www.yatego.com/getraenke-treitz/p,4b265058c26ba,4b2268bfd899d4_6,molinari-sambuca-extra-1-00l
· https://en.wikipedia.org/wiki/Ethanol
· http://www.elecs.co/product/omron-digital-thermometer-mc-342fl-ladies-oral-odt05-510.html
·
Дата добавления: 2015-10-28; просмотров: 102 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Фоточки запили чтоле | | | Шон Коннери, 81 год |