
Читайте также:
|
Koroleva O.N.
Institute of mineralogy UrB RAS, Miass, Russia
koroleva@mineralogy.ru
Structural studies of silicate systems are important for geochemists as they promote understanding of magmatic processes. Melt composition and particularly overall degree of polymerization is a major factor governing melt structure and properties. The local structure of silicate system can be presented as a set of silicon-oxygen tetrahedrons with various proportions of bridging and non-bridging oxygen atoms (Qn-units, where n is a number of bridging oxygen atoms). Dependence of melt structure from composition can be determined as a Qn – distribution vs. modifier cation content. Alkali silicate melts, especially sodium containing system, are convenient model systems due to their low melting points. Lithium silicate melts haven’t been investigated properly before in a spread region of compositions despite these melts and their glasses have unique features [1].
The distribution of structural units Qn can be determined experimentally by techniques of magic-angle spinning nuclear magnetic resonance (MAS-NMR) or Raman spectroscopy. However NMR spectroscopy can be applied only to study overcooled melts, because the NMR spectra of various structural units Qn are averaged during melting and become indiscernible. While, Raman spectroscopy has no such restrictions in terms of temperature, so that in situ structural studies can be carried on in melts [2-4].
This work is devoted to synthesis and vibrational spectroscopy of two compositional series. Glasses of хLi2O·(100-х)SiO2, where x = 33, 40, 50, 55, 60 mol.% and хNa2O·(100-х)SiO2, where х = 33, 40 и 50, 55, 60 and 67 mol.% composition, were synthesized from lithium (sodium) carbonate and SiO2. Dependence of silicate melt structure from modifier cation content have been studied by Raman spectroscopy in temperature range up to 1462 К. High-temperature Raman spectra were collected with an experimental devise based on a DFS-24 spectrometer [5]. The spectra were exited and registered by a powerful LTI-701 Nd laser (λ = 532 nm, (P) = 1 W) with a pulse frequency of 8.7 kHz, which was used coupled with a synchronized photon counter that was opened only during a laser pulse. To compare spectra obtained at various temperatures, they were adjusted for the thermal occupancy of vibration levels.
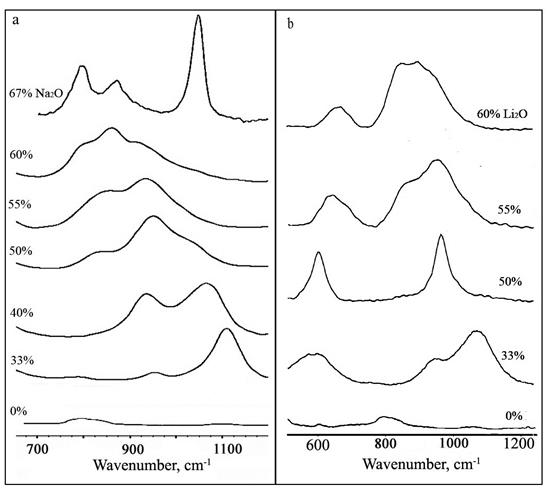
Fig. Raman spectra of melts of composition хNa2O·(100-х)SiO2, where х = 0, 33, 40, 50, 55, 60 and 67 mol.% (a) and хLi2O·(100-х)SiO2, where x = 0, 33, 50, 55, 60 mol.% (b) recorded at 1300 K.
The figure shows changes in high-frequency region (800-1200 см-1) of Raman spectra of melts of the composition хNa2O·(100-х)SiO2 (a) and хLi2O·(100-х)SiO2 (b) recorded at 1300 K. Liquid silicates on small addition of alkaline metal oxide shows a band near 1050-1100 см-1. The band relative intensity increases and maximizes near the disilicate (33% M2O) composition. At the same time a new band near 930-950 см-1 is appeared with an increase of a modifier oxide content. An intensity of the first band decreases, and the second - increases and reaches the maximum in metasilicate melt spectrum (50% M2O) at the further increase of metal oxide concentration. Except these two bands the bands in the field of 900 and 850 cm-1 are observed in a Raman spectra with the raised cation modifiers content. Their intensities are maximum in composition of pyro- and orthosilicate, corresponding. It is necessary to notice, that there is an intensive band near 1050 cm-1 corresponding to vibrations of  ions in Raman spectra of sodium silicate melts with mole fraction 67% of Na2O.
ions in Raman spectra of sodium silicate melts with mole fraction 67% of Na2O.
The fitting procedure was made taking into account the second coordination sphere of a Si atom. For this purpose, the high-frequency region of the spectrum was represented as a superposition of the Gaussian forms. To obtain the correct interpretation of all bands in Raman spectra, an additional intermediate types of structural unites Qn’ were entered into description of melt structure. It has allowed us to describe properly changes of Raman spectra depending on composition. It was shown that regularities of melts anion structure formation for Li2O·SiO2 and Na2O·SiO2 systems are simillar. The main anion groupings existing in systems are defined and structural changes vs. composition are determined. Though the changes of polimerisayion degree depending the composition of glasses and melts are similare for Li and Na systems, but temperature dependence of Qn-distribution in these systems are different.
The work was supported by the Grant of Ural Branch of RAS grant for young scientific leaders and the Grant of President of Russian Federation (МК-109.2011.5).
References:
1 Mysen B.O. and Frantz J.D., (1994) Silicate Melts at Magmatic Temperatures - in-Situ Structure Determination to 1651-Degrees-C and Effect of Temperature and Bulk Composition on the Mixing Behavior of Structural Units, Contributions to Mineralogy and Petrology, 117, 1-14 pp.
2 Mysen B.O. and Frantz J.D., (1993) Structure of Silicate Melts at High-Temperature - in-Situ Measurements in the System Bao-Sio2 to 1669-Percent-C, American Mineralogist, 78, 699-709 pp.
3 Anfilogov V.N., Bykov V.N. and Osipov A.A., eds., Silicate Melts (Moscow: Nauka, 2005).
4 Malfait W.J., Zakaznova-Herzog V.P. and Halter W.E., (2007) Quantitative Raman Spectroscopy: High-Temperature Speciation of Potassium Silicate Melts, Journal of Non-Crystalline Solids, 353, 4029-42 pp.
5 Bykov V.N., Osipov А.А., Anfilogov V.N., (1997) High-temperaure apparatus for Raman spectra registration, Melts, № 4, pp. 28-31.
Дата добавления: 2015-10-29; просмотров: 121 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Growth, morphology and optical properties of γ-BiB3O6 single crystals | | | Micro-FTIR study of quartz crystals from Zhelannoye deposit, Subpolar Urals |