
Читайте также:
|
The major heavy metals (metals with relatively high atomic weight) that pose health hazards to people and ecosystems include mercury, lead, cadmium, nickel, gold, platinum, silver, bismuth, arsenic, selenium, vanadium, chromium, and thallium. Each of these elements may be found in soil and water that has not been contaminated by people. However, each of these metals has uses in the modern industrial society, and each is also a by-product of the mining, refining, and use of other elements. Heavy metals often have direct physiological toxic effect. Some are stored or incorporated in living tissue, sometimes permanently. Heavy metals tend to accumulate over time in fatty body tissue. As a result, a little arsenic each day may eventually result in a fatal dose (the plot of more than one murder mystery).
Mercury, thallium, and lead are very toxic to people. They have long been mined and used, and their toxic properties are well known. Mercury, for example, is the “Mad Hatter” element. At one time, it was used in making felt hats stiff, and because mercury damages the brain, hatters were known to act peculiarly in Victorian England. Thus, the Mad Hatter in Lewis Carroll’s Alice in Wonderland had real antecedents in history.
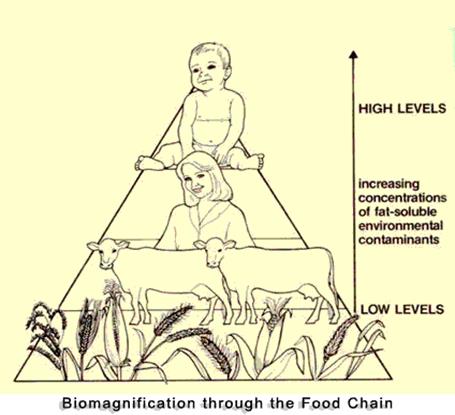
Chemical elements released from rocks or human processes can become concentrated in humans through many pathways. These pathways may involve what is known as biomagnification – the accumulation or increase in the concentration of a substance in living tissue as it moves through a food web (also known as bioaccumulation). For example, cadmium, which influences the risk of heart disease, may enter the environment via ash from burning coal. The cadmium in coal exists in very low concentrations. After coal is burned in a power plant, the ash is collected in a solid form and disposed in a landfill (свалка). The landfill is covered with soil and revegetated. The low concentration of cadmium in the ash and soil is taken into the plants as they grow. But the concentration of cadmium in the plants is three to five times greater than the concentration in the ash. As cadmium moves through the food chain, it becomes more and more concentrated. By the time it is incorporated in the tissue of people and other carnivores, the concentration is approximately 50-60 times the original concentration in coal.
Mercury in aquatic ecosystems offers another example of biomagnification. Mercury is a potentially serious pollutant of aquatic ecosystem, such as ponds, lakes, rivers, and the ocean. Natural sources of mercury in the environment include volcanic eruption and the erosion of natural mercury deposits. However, we are most concerned with human input of mercury into the environment through burning coal in power plants, incinerating (сжигать) wastes, and processing metals, such as gold. Although we are unable to measure it precisely, it is estimated that human activities have doubled or tripled the amount of mercury in the atmosphere, and that it is increasing at about 1.5 % per year.
A major source of mercury in many aquatic ecosystems is precipitation from the atmosphere – rain and snow. Most of what is deposited is inorganic mercury, but once this mercury is in surface water, a process known as methylation may occur. Methylation changes inorganic mercury into methyl mercury through bacterial activity. Methyl mercury is much more toxic than organic mercury, and it is eliminated more slowly from animals’ system.
As the methyl mercury works its way through food chain, biomagnification occurs, so that higher concentrations of methyl mercury are found farther up the food chain. Thus, big fish that eat little fish contain higher concentration of mercury than do smaller fish and the aquatic insects that the fish feed on. Large fish, such as tuna and swordfish, have elevated mercury concentrations, which is why today we are advised to limit our consumption of these fish. Indeed, pregnant women are advised not to eat them at all.
From E. A. Keller, D.B. Botkin. Essential Environmental Science. John Wieley & Sons, Inc., 2007.
1. What is biomagnification? Describe its process looking at the picture.
Дата добавления: 2015-10-26; просмотров: 83 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Tell about the greenhouse effect using this picture | | | Give the examples of biomagnification of some elements. |