
|
Читайте также: |
A hydrogen atom has one electron which "rotates" in a nuclear field. An electric force on Coulomb attraction acts between the electron and the nucleus. The potential energy of an electron in a nuclear field is
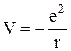 , (11.1)
, (11.1)
where e is the charge of an electron and r is the distance between the nucleus and electron. Such an atom constitutes a peculiar kind of potential well and is illustrated in fig.11.1.
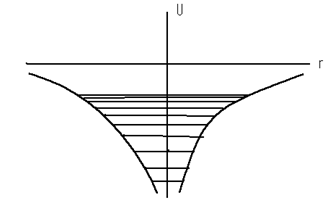
Figure 11.1
The electron inside the atom has a negative potential energy since the minimum value of potential energy tends to infinity when r → 0 and the maximum value is equal to zero. Fig. 11.2 shows the energy levels obtained from the solution of the Schrodinger equation
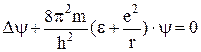 . (11.2)
. (11.2)
 |
Figure 11.2
An important feature of the solution is the drawing together of the levels as the quantum number n increases. The scales of values, which are proportional to energy are given in the units adopted in spectroscopy: volts and reciprocal centimetres. The energy level formula may by written in the form
 . (11.3)
. (11.3)
For historical reasons, it is customary to write this formula in the for
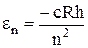 , (11.4)
, (11.4)
where
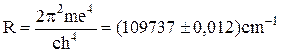 (11.5)
(11.5)
is the Rydberg’s constant.
The atomic electron may be located at any one of n levels. The energy of a free hydrogen atom on which no force acts is at the lowest energy level
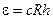 , (11.6)
, (11.6)
The energy ε = cRh is called the ionisation energy. If the energy imparted to hydrogen atom is less than cRh, a transition of the atom occurs to one of the n levels. Such an atom is said to be in an exited state.
An atom stays in an excited state for a small fraction of a second and then passes to a lower level with the emission off a photon in accordance with the equation
hνmn = εm - εn = cRh (1/n2 - 1/m2). (11.7)
By calculating for a given n the ν frequencies corresponding to the numbers m = n +1, n +2,..., we obtain a series of frequencies of lines in the hydrogen spectrum. The series corresponding to n = 2 is known as the Balmer series.

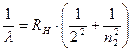 . (11.8)
. (11.8)
Дата добавления: 2015-10-29; просмотров: 138 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| LABORATORY WORK № 61 | | | Теоретична частина |