
O192,6 - 191,5 173,51 188,72 DSo, 298 J/mol
· Call the substances. Calculate the equivalent weights for the following substances: NO, H3PO4, Hg(NO3)2, Mn2O3, H2CO3, Ca(H2PO4)2
· To determine the equilibrium temperature at which in system there comes an equilibrium state at 1500 K
· SiCl4(g) + 2H2O(g) = SiO2(s) + 4HCl(g)
DSo, 298 J/mol
· Calculate Colligative Properties for the solution of Cd(NO3)2 under standard conditions (Mass fraction 5 (%), density 1,015 g/ml).
· Write the equations of the below given chain.
· Calculate concentrations (mole fraction, molar, normal, molal and titration) and Colligative Properties for the following solutions of Al(NO3)3 under standard conditions (Mass fraction 5 (%), density 1,015 g/ml).
· Define an oxidizer and a reducer, work out the equation of electronic balance, place factors.
· 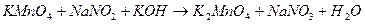
· To define a reaction orientation
· Call the substances. Calculate the equivalent weights for the following substances: SO2, K2HPO4, Ag2SO4, Mn2O3, H2CO3, Ca(H2PO4)2
· Calculate concentrations (mole fraction, molar, normal, molal and titration) for the solution of CuSO4 under standard conditions (Mass fraction 10%, density 1,019 g/ml).
· Call the substances. Calculate equivalent weight and equivalent volume of substance.: Al2O3, HNO3, NH4Cl, SnO2, Fe(OH) 2, CuSO4
· How many milliliters of 30 % of HCl solution (ρ=1.15 g/ml) are necessary to prepare 3 L of its solution with CN=0.1 mol/L?
· How many milliliters of 50 % of sulfuric acid (ρ=1.4 g/ml) must be diluted in water to get 10 L of its solution (CN=0.25 mol/L)?
· What is the osmotic pressure of 5 % NaHCO3solution that is used for the injection during acidosis (ρ=1.035, α=0.98)?

· Call the substances. Calculate the equivalent weights for the following substances: CO2, HNO2, Ca3(PO4)2, SO3, KHS, Pb(NO3)2
· Standard enthalpy formation of CO2equals -393.6 kJ/mol, H2O equals -285.9 kJ/mol, C6H12O6is -1272.45 kJ/mol. Calculate the standard enthalpy change for the reaction
· C6H12O6+ O2®CO2+ H2O.
· Standard enthalpy formation of HCl equals -92.05 kJ/mol, HI equals -25.1 kJ/mol. Calculate the standard enthalpy change for the reaction
· What is the osmotic pressure of 18 % of sucrose at 200C if the solution density is 1,07 g/cm3?
· Call the substances. Calculate the equivalent weights for the following substances: K2O, H2SO4, Al(OH)2Cl, SiO2, Mn(OH)2, Al(OH)Br2
· How many grams of Н3РО4 must be taken for preparation of 0.2 M solution in the volume of 5 L?
· Call the substances. Calculate the equivalent weights for the following substances: MgO BaO, H2ZnO2, FeS, CrO, NH4OH, Fe(HSO4)2
· What is the osmotic pressure of 18 % of sucrose at 200C if the solution density is 1,07 g/cm3?
Дата добавления: 2015-10-29; просмотров: 121 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Lowering Vapor Pressure | | | Reactions with water |