
Читайте также:
|
Solutions
Solution. Solute. Solvent. Solubility. Parts-per concentration. Molarity: mole/volume bas is. Normality and equivalents. Mole fraction: mole/mole basis. Conversion between concentration measures
Solutions are homogeneous (single-phase) mixtures of two or more components. Particles of one substance are dissolved and scrambled among the particles of another substance.
Solvent: the major part of solution, dissolves the solute (usually liquid and usually water).
Salute: the part of solution that is being dissolved.
| State of solution | State of solvent | State of Solute | Exemples |
| Gas | Gas | Gas | Air |
| Liquid | Liquid | Gas | Oxygen in water |
| Liquid | Liquid | Liquid | Alcohol in water |
| Liquid | Liquid | Solid | Salt in water |
| Solid | Solid | Gas | Hydrogen in palladium |
| Solid | Solid | Liquid | Mercury in silver |
| Solid | Solid | Solid | Silver in gold |
Concentrated solutions contain lots of solute per amount of solution. Dilute solutions contain less solute per amount of solution.
An unsaturated solution has less than the maximum amount of dissolved solute. A saturated solution has the maximum amount of dissolved solute.
Supersaturated: describes a solution that contain more than the maximum amount of solute that can possibly be dissolved at a specified temperature.
Solubility: a measurement of the amount of solute than can be dissolved in a solvent at a specified temperature.
Concentration is a general term that expresses the quantity of solute contained in a given amount of solution. Various ways of expressing concentration are in use; the choice is usually a matter of convenience in a particular application. You should become familiar with all of them.
Percent (%)
This is the mass of the solute divided by the mass of the solution (mass of solute plus mass of solvent), multiplied by 100.
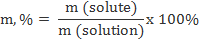
Volume percent or volume/volume percent most often is used when preparing solutions of liquids. Volume percent is defined as:

Mole Fraction (X)
This is the number of moles of a compound divided by the total number of moles of all chemical species in the solution. Keep in mind, the sum of all mole fractions in a solution always equals 1.
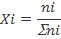
Molarity (M)
Molarity is probably the most commonly used unit of concentration. It is the number of moles of solute per liter of solution (not necessarily the same as the volume of solvent!).

Molality (m)
Molality is the number of moles of solute per kilogram of solvent. Because the density of water at 25°C is about 1 kilogram per liter, molality is approximately equal to molarity for dilute aqueous solutions at this temperature.
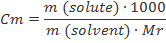
Normality (N)
Normality is equal to the gram equivalent weight of a solute per liter of solution. A gram equivalent weight or equivalent is a measure of the reactive capcity of a given molecule. Normality is the only concentration unit that is reaction dependent.
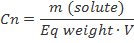
Сolligative properties are properties of solutions that depend upon the ratio of the number of solute particles to the number of solvent molecules in a solution. They are independent of the nature of the solute particles, and are due essentially to the dilution of the solvent by the solute. The word colligative is derived from the Latin colligatus meaning bound together, since these properties are bound together by the fact that they all depend on the number of solute particles and not on the type of chemical species present. This number can be related to the various units for concentration of solutions.
Colligative properties include:
1. Relative lowering of vapor pressure
2. Elevation of boiling point
3. Depression of freezing point
4. Osmotic pressure.
Lowering Vapor Pressure
Vapor Pressure Depression
Very few of the physical properties of a solution are colligative properties. The vapor pressure of the solvent when we add a solute to form a solution. We'll define Po as the vapor pressure of the pure liquid -- the solvent -- and P as the vapor pressure of the solvent after a solute has been added.
Po = vapor pressure of the pure liquid, or solvent
P = vapor pressure of the solvent in a solution
When the temperature of a liquid is below its boiling point, we can assume that the only molecules that can escape from the liquid to form a gas are those that lie near the surface of the liquid. When a solute is dissolved in a solvent, the number of solvent molecules near the surface decreases, and the vapor pressure of the solvent decreases.
The vapor pressure of the solvent escaping from a solution should be smaller than the vapor pressure of the pure solvent. P<Po.
Raoult's law. The varoup pressure of a solution of a non-volatile solute is equal to the vapour pressure of the pure solvent at that temperature multiplied by it's mole fraction.
• Non-volatile solutes reduce the ability of the surface solvent molecules to escape the liquid.
• Therefore, vapor pressure is lowered.
• The amount of vapor pressure lowering depends on the amount of solute.
In equation form this reads: РА=c∙Р0А
Where: PA = vapor pressure with solute, PA ° = vapor pressure without solute (pure solvent), and cA = mole fraction of A.
The change in boiling point is proportional to the number of solute particles present and can be related to the molality of the solution:
DTb = Kb.m
where DTb = boiling point elevation
Kb = molal boiling point elevation constant
m = molality of solution
The magnitude of the freezing point depression is proportional to the number of solute particles and can be related to the molality of the solution.
D Tf = Kf ž m
where DTf = freezing point depression
Kb = molal freezing point depression constant
m = molality of solution
Normal Boiling point and Normal Freezing Point for several Solvents:
| Solvent | Normal Boiling point, 0C | Kb | Normal Freezing Point, 0C | Kf |
| Water, H2O | 0.52 | 0.0 | 1.86 | |
| Benzene, C6H6 | 80.1 | 2.53 | 5.5 | 5.12 |
| Ethanol, C2H5OH | 78.4 | 1.2 | -114.6 | 0.99 |
| Carbon tetrachloride, CCl4 | 76.8 | 5.02 | -22.3 | 29.8 |
| Chloroform, CHCl3 | 61.2 | 3.6 | -63.5 | 0.68 |
Methods of Transport Across Membranes:
1. Diffusion -passive transport - no energy expended.
2. Osmosis - Passive transport of water across membrane.
Molecules in solution tend to slowly spread apart over time. This is diffusion. Diffusion will continue until equilibrium is reached. This means there will be an equal distribution of molecules throughout the space. This is why food coloring moves throughout a beaker of water; why odors smell strong at first and then disappear over time. Equilibrium, a result of diffusion, shows the uniform distribution of molecules of different substances over time as indicated in the above diagram.
The osmotic pressure of a solution is the difference in pressure between the solution and the pure liquid solvent when the two are in equilibrium across a semipermeable membrane, which allows the passage of solvent molecules but not of solute particles. If the two phases are at the same initial pressure, there is a net transfer of solvent across the membrane into the solution known asosmosis. The process stops and equilibrium is attained when the pressure difference equals the osmotic pressure.
Two laws governing the osmotic pressure of a dilute solution were discovered by the German botanist W. F. P. Pfeffer and the Dutch chemist J. H. van’t Hoff:
1. The osmotic pressure of a dilute solution at constant temperature is directly proportional to its concentration.
2. The osmotic pressure of a solution is directly proportional to its absolute temperature.
Дата добавления: 2015-10-29; просмотров: 154 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Задания для закрепления | | | DSo, 298 J/mol |