
|
Читайте также: |
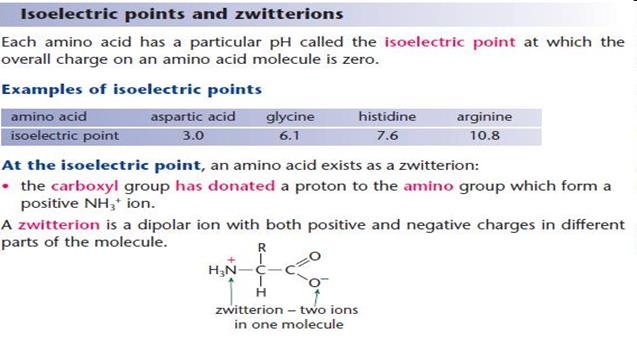
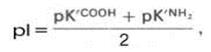
Amino acids are organic-heterofunctional compounds containing both amino and carboxy groups.
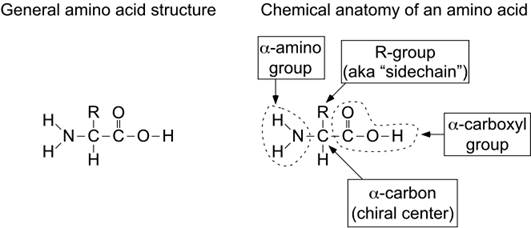
|
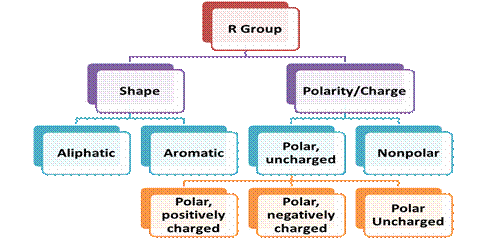
1. On the structure of the side radicals
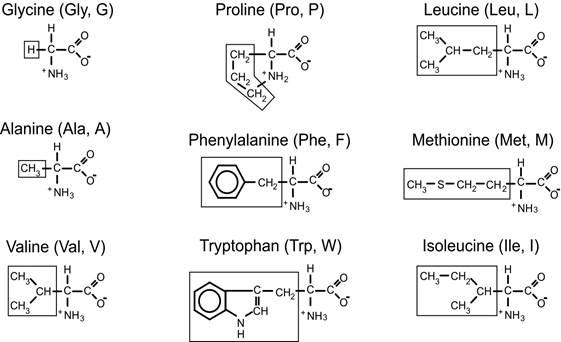
- Acyclic (15 amino acids);
- Cyclic (heterocyclic, homocyclic, aromatic)
2. On the polarity and charge of the radical
- Nonpolar, hydrophobic (9)
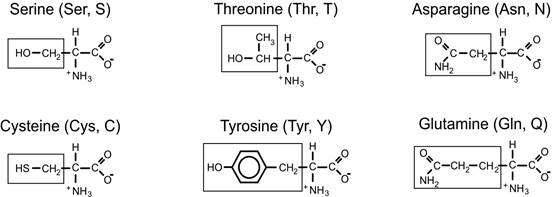
- Polar but uncharged (6)
- Polar, negatively charged (2)
- Polar, positively charged (3)
Non-polar amino acids
Polar, non-charged amino acids
Negatively-charged amino acids
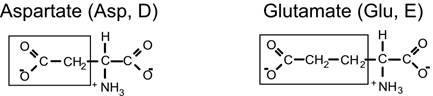
Positively-charged amino acids
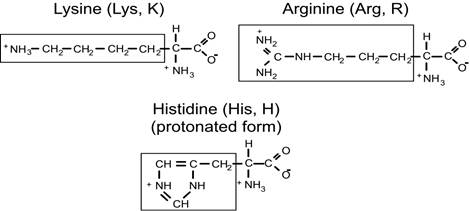

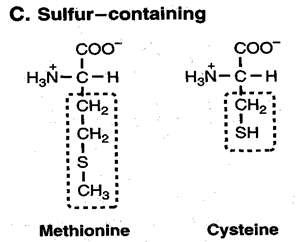
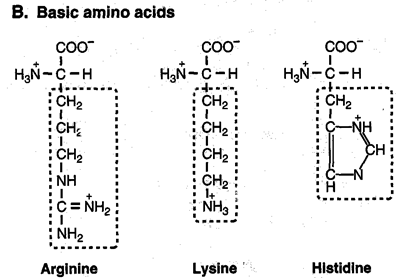
3. On the number of amino - and carboxyl groups
- mono-amino-monocarboxyl (15);
- mono-amino-di-carboxyl (2);
- di-amino-mono-carboxyl(3);
4. On nutritional value
- interchangeable (non-essential);
- indispensable (essential);
- partially interchangeable (Arg, His);
- relatively interchangeable (Tyr, Cys);
Amino Acids:The building blocks of proteins
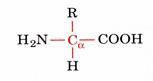
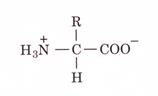 alfa amino acids because of the a carboxylic and a amino groups
alfa amino acids because of the a carboxylic and a amino groups
pK1 and pK2 respectively pKR is for R group pK’s
pK1» 2.2 while pK2» 9.4
In the physiological pH range, both carboxylic and amino groups are completely ionized
Optical activity - The ability to rotate plane - polarized light
Asymmetric carbon atom
Chirality - Not superimposable
Mirror image - enantiomers 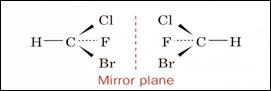
(+) Dextrorotatory - right - clockwise
(-) Levorotatory - left counterclockwise
Na D Line passed through polarizing filters 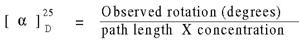
One or many chiral centers
N chiral centers 2N possible stereoisomers and 2N-1 are enantiomeric
For N = 2 there are 4 possible sterioisomers of which 2 are enatiomers and 2 are diastereomers. Diastereomers are not mirror images and have different chemical properties.
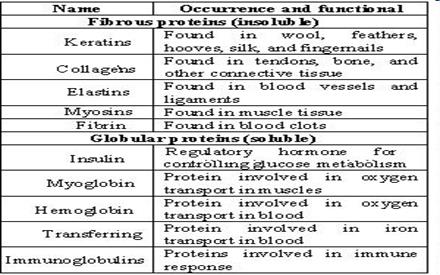
4. Classification of proteins: simple and complex proteins. Globular and fibrous proteins. Characteristic. Examples.
Classification of proteins:
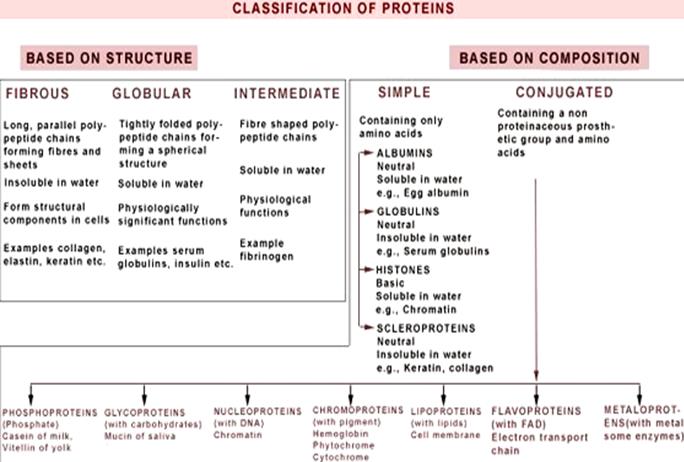
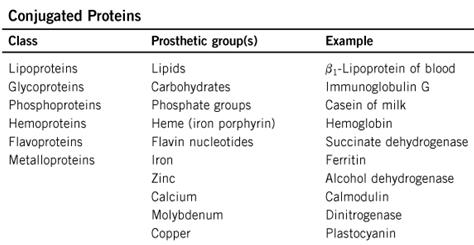
Globular proteins are:
enzymes, antibodies, many hormones, toxins, vectors of different compounds. For example, enzymes, trypsin, ribonuclease, pepsin, carrier proteins – albumin (fatty acids), hemoglobin (molecular oxygen).
Fibrous: Left-twisted superhelix of collagen,formed of three polypeptide chains,that overlap around a common axis(3-wire rope).
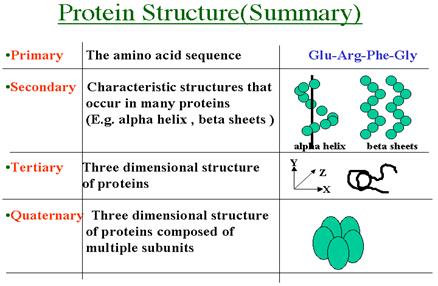
5. Structural organization of protein molecules: primary, secondary, tertiary, quaternary structure of the protein. Forces that stabilize each of the levels.
Primary structure - an order of amino acids in the protein chain. Primary structure is a sequence of amino acids in polypeptide chain. Amino Acids Are Joined By Peptide Bonds In Peptides
- a-carboxyl of one amino acid is joined to a-amino of a second amino acid (with removal of water)
- only a-carboxyl and a-amino groups are used, not R-group carboxyl or amino groups.
The secondary structure of a protein results from hydrogen bonding between amino acids in the peptide chain. This leads to twisting or folding of the chain into the alpha helix and the beta pleated sheet shapes. Secondary Structure: The way in which the primary structure of a polypeptide chain folds.
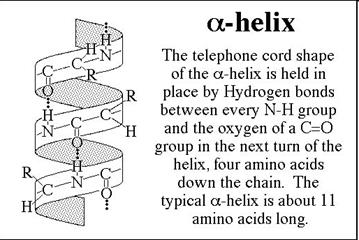 • In the a-helix, the carbonyl oxygen of residue “i” forms a hydrogen bond with the amide of residue “i+4”.
• In the a-helix, the carbonyl oxygen of residue “i” forms a hydrogen bond with the amide of residue “i+4”.
• Although each hydrogen bond is relatively weak in isolation, the sum of the hydrogen bonds in a helix makes it quite stable.
• The propensity of a peptide for forming an a-helix also depends on its sequence.
• In a b-sheet, carbonyl oxygens and amides form hydrogen bonds.
• These secondary structures can be either antiparallel (as shown) or parallel and need not be planar (as shown) but can be twisted.
• The propensity of a peptide for forming b-sheet also depends on its sequence. 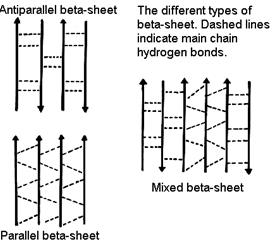
• betta-turns allow the protein backbone to make abrupt turns.
• Again, the propensity of a peptide for forming b-turns depends on its sequence.
The tertiary sructure of a protein can be thought of as the overall, unique, three dimensional folding of a protein. Tertiary structure - global folding of a protein chain
Stabilizing Forces: 1. Electrostatic/ionic,2. Hydrogen bonds,3.Hydrophobic interaction, 4. Disulfide bonds 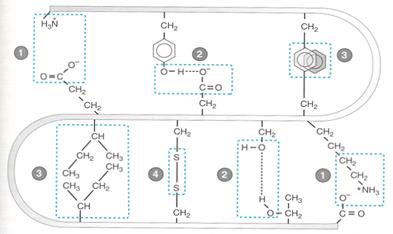
Quaternary structure of protein: Proteins with multiple polypetide chains are oligomeric proteins. The structure formed by monomer-monomer interaction in an oligomeric protein is known as quaternary structure. Oligomeric proteins can be composed of multiple identical polypeptide chains or multiple distinct polypeptide chains. Proteins with identical subunits are termed homo-oligomers. Proteins containing several distinct polypeptide chains are termed hetero-oligomers.
6. Denaturation of proteins. Factors of denaturation (examples).
Denaturation -process where proteins lose their tertiary structure and secondary structure from some factors.
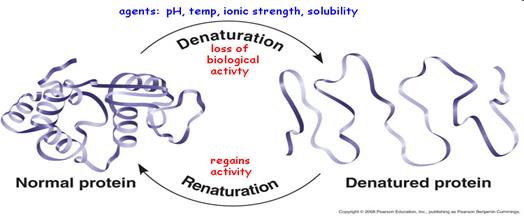
Factors: 1. Physical (temperature, radiation ioniziruschee, pH, ultrasound)
2. Chemicals (salts of copper, mercury, arsenic, iron, concentrated inorganic acid - HNO3, H2SO4, HCl, organic acids, alkaloids - tannin, urea, organic solvents)
3. Biological (enzymes, pepsin)
7. Specific reaction for some aminoacids (1 laboratory work).
1. Biuret’s Test: The Biuret Test positively identifies the presence of proteins (not less than two peptides). The reaction in this test involves the complex formation of the proteins with Cu2+ ions in a strongly alkaline solution. This reaction is qualitative and based on the presence of peptide bonds in proteins. Biuret reaction is allowed to identify not only proteins, peptides and polypeptides, but biuret (NH2-CO-NH-CO-NH2), oxamide (NH2-CO-CO-NH2), histidine. The intensity of the color depends on the amount of protein in solution. This allows to use this reaction for the quantitative determination of a protein. Color of the solution depends on the length of the polypeptide chain. Proteins give a blue-violet color; their hydrolysis products (poly-and oligo-peptides) – are red or pink in color.
2. Xanthoproteic Test: This reaction indicates the presence of aromatic amino acids - tyrosine, phenylalanine, tryptophan in proteins. In the presence of concentrated nitric acid, the aromatic phenyl ring gets nitrated to give yellow colored nitro-derivatives. At alkaline pH the color changes to orange due to the ionization of the phenolic group. Some amino acids contain aromatic groups that are derivatives of benzene. These aromatic groups can undergo reactions that are characteristics of benzene and benzene derivatives. One such reaction is the nitration of a benzene ring with nitric acid. The amino acids that have activated benzene ring can readily undergo nitration. This nitration reaction, in the presence of activated benzene ring, forms yellow product.
3. Ninhydrin Test: In this reaction, protein solutions, polypeptides, peptides and free α-amino acids by heating with ninhydrin give blue, blue-purple or pink-violet color. Colouring in this reaction develops at the expense of α-amino groups. The amino acids proline and hydroxyproline also react with ninhydrin, but they give a yellow colored complex instead of a purple one subjected to the ninhydrin reaction. This reaction provides an extremely sensitive test for amino acids.
4. Millon’s Test: Millon’s test is specific to phenol containing structures (tyrosine is the only common phenolic amino acid). Millon’s reagent is concentrated HNO3, in which mercury is dissolved. As a result of the reaction a red precipitate or a red solution is considered as positive test. A yellow precipitate of HgO is NOT a positive reaction but usually indicates that the solution is too alkaline. A brick red color is a positive reaction.
5. Foll’s Test: (sulfur-containing AA): Sulphur containing amino acids, such as cysteine and cystine upon boiling with sodium hydroxide (hot alkali) yield sodium sulphide. This reaction is due to partial conversion of the organic sulphur to inorganic sulphide, which can detected by precipitating it to lead sulphide, using lead acetate solution. - A brown or black color is a positive test for sulfides.
6. Adamkewich’s Test: (for Trp) Proteins containing tryptophan, in the presence of glyoxylic acid and sulfuric acids give a red -violet color. The reaction is based on the ability of tryptophan to interact in an acidic medium with aldehydes (glyoxylic acid) to form a colored condensation products. After 5-10 minutes at the interface of the two layers are watching the formation of red-purple ring.
8. General principles of the enzymes structure. (holoenzyme, apoenzyme, coenzyme, prosthetic group). Functions of cofactor and apoenzyme.
• Enzymes are biological molecules that catalyze (i.e., increase the rates) of chemical reactions. Substrate - molecule, which is transformed during the chemical reaction.
• Inhibitors are molecules that decrease enzyme activity;
• Activators are molecules that increase the enzyme activity.
• Prosthetic group - a metal or other co-enzyme (non-protein part) covalently bound to enzyme
• Active site - a region of an enzyme comprised of different amino acids where catalysis occurs (determined by the tertiary and quaternary structure of each enzyme)
• Co-factor - organic or inorganic molecules that are required by some enzymes for activity. These include Mg2+, Fe2+, Zn2+ and larger molecules termed co-enzymes like nicotinamide adenine dinucleotide (NAD+), coenzyme A, and many vitamins.
• Holoenzyme - a complete, catalytically active enzyme including all co-factors
• Apoenzyme - the protein portion of a holoenzyme minus the co-factors
Each enzyme has its own unique protein structure and shape, which is designed to match or COMPLEMENT its substrate. 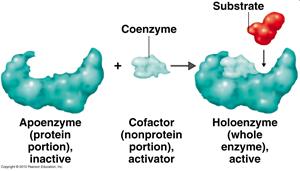
9. Coenzymes: structure, functions of coenzymes.
Coenzymes: smaller molecules that aid in enzyme chemistry.
Enzymes can:
a. Carry out acid-base reactions
b. Transient covalent bonds
c. Charge-charge interactions
Enzymes can not do:
d. Oxidation -Reduction reactions
e. Carbon group transfers
Дата добавления: 2015-10-31; просмотров: 532 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Section 4. escapism | | | Substrate concentration: Enzymic reactions |