
Читайте также:
|
The magnetic quantum number is designated as:
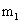
This number describes how the various orbitals are oriented in space. The value of this number depends on the value of l. The values allowed are integers from – l to 0 to + l. For example, if the value of l = 1 (p orbital), you can write three values for this number: –1, 0, and +1. This means that there are three different p subshells for a particular orbital. The subshells have the same energy but different orientations in space.
The second row (b) of the figure shows how the p orbitals are oriented in space. Notice that the three p orbitals correspond to magnetic quantum number values of –1, 0, and +1, oriented along the x, y, and z axes.
Дата добавления: 2015-08-03; просмотров: 52 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| The angular momentum quantum number | | | The spin quantum number |