
|
Читайте также: |
Two models of atomic structure are in use today: the Bohr model and the quantum mechanicalmodel. The quantum mechanical model is based on mathematics. Although it is more difficult to understand than the Bohr model, it can be used to explain observations made on complex atoms.
A model is useful because it helps you understand what’s observed in nature. It’s not unusual to have more than one model represent and help people understand a particular topic.
The quantum mechanical model is based on quantum theory, which says matter also has properties associated with waves. According to quantum theory, it’s impossible to know the exact position and momentum of an electron at the same time. This is known as the Uncertainty Principle.
The quantum mechanical model of the atom uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron. So, this model is based on probability rather than certainty.
Four numbers, called quantum numbers, were introduced to describe the characteristics of electrons and their orbitals:
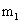
Дата добавления: 2015-08-03; просмотров: 63 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Aseptic void | | | The angular momentum quantum number |