
|
Читайте также: |
| Fe | Co | Ni | |
| density, g/сm3 | 7.87 | 8.90 | 8.91 |
| tm, oС | |||
| Rа, nm | 0.125 | 0.125 | 0.124 |
| Ionisation energy Е ® Е+, kJ∙mol-1 | 762.5 | 760.4 | 737.1 |
| Е+ ® Е2+, kJ∙mol-1 | 1561.9 | 1753.0 | |
| Е2+ ® Е3+, kJ∙mol-1 | |||
| Еo(Е2+ + 2е = Е), V | -0.44 | -0.29 | -0.25 |
Iron is a fairly soft metal, existing in different forms according to temperature (Fig. 1):
a-Fe 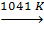 b-Fe
b-Fe 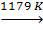 g-Fe
g-Fe 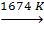 d-Fe.
d-Fe.
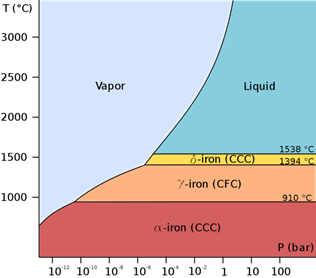
Figure 1. Phase diagram of pure iron
Alpha iron, also known as ferrite, is the most stable form of iron at normal temperatures. It is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).
Above 912 °C and up to 1400 °C α-iron undergoes a phase transition from bcc to the fcc configuration of γ-iron, also called austenite (Fig. 2). This one is similarly soft and metallic but can dissolve considerably more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is used in the type of stainless steel used for making cutlery, and hospital and food-service equipment.
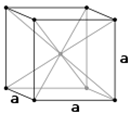

Figure 2. The body centered cubic unit cell (bcc or ccc) of a-Fe and face-centered cubic unit cell (fcc) of γ-Fe
Magnetic properties of iron [1]. Below t = 770°С it’s ferromagnetic substance (this temperature of ferro(ferri-)magnetic-paramagnetic transition is called the Curie point), in the range 770-910 оС it’s paramagnetic substance (b-Fe). At 910-1401°С it’s a paramagnetic (g-Fe) with a face-centred cubic lattice. At 1401-1539°С (b.p.) it’s d-Fe with body-centred cubic lattice.
Дата добавления: 2015-07-25; просмотров: 87 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| УСТАНОВКА. | | | Iron triad trends |