
|
Читайте также: |
Ti, Zr, and Hf have higly negative values of standard electrode potential, Е0, that characterises equilibrium between metallic and ionic forms of the element:
| Ox/Red | Ti3+/Ti | Zr4+/Zr | Hf4+/Hf |
| Е0, V | -1.76 | -1.43 | -1.57 |
However, despite the strong reducing properties elements of titanium subgroup are chemically stable at normal conditions due to protective properties of oxide film ЕО2.
Reactions with nonmetals require heating:
E + О2 = EО2
E + 2Hal2 = EHal4
Ti(Zr) + 2S = Ti(Zr)S2
Fine powders of metals of titanium subgroup are pyrophoric. Ti, Zr, and Hf are stable in some corrosive media (moisten air, sea water, solutions of salts), and also in most of acids except HF, hot H3PO4, and alkalis. For example, cold HNO3 passivates Ti, Zr, and Hf due to increase of oxide film thickness. Zr and Hf don’t react with HCl and diluted H2SO4. Hot HCl and diluted H2SO4 interaction with Ti is shown below:
2Ti + 6HCl = 2TiCl3 + 3H2
2Ti + 3H2SO4 = Ti2(SO4)3 +3H2
Aquacomplex [Ti(H2O)6]3+ that appears is lilac.
Hot nitric acid reacts with titanium too:
3Ti + 4HNO3 + H2O = 3H2TiO3 + 4NO.
Generally, dissolution and simultaneous oxidation of Ti subgroup elements in acid medium depends on the fact whether their oxidation and formation of Е(IV) anions (as a rule anionic coordination compounds) are possible.
2Ti + 6HF ® 2TiF3 + 3H2
Zr(Hf) + 6HF = H2[Zr(Hf)F6] + 2H2
3Е + 4HNO3 + 18HF = 3H2[ЕF6] + 4NO + 8H2O
Zr + 5H2SO4 = H2[Zr(SO4)3] + 2SO2 + 4H2O
3Zr + 4HNO3 + 18HCl = 3H2[ZrCl6] + 4NO + 8H2O
Titanium subgroup elements are soluble in molten alkalis
Е + 4NaOH = Na4ЕO4 + 2H2
Hf is soluble in molten KHF2:
Hf + KHF2 = K2HfF6 + 2KF + 2H2
Ti, Zr і Hf form anionic coordination compounds. In the series Ti—Zr—Hf coordination number is increased: (М1-cations of monovalent metals) М12[TiF6], М12[ZrF6], М13[ZrF7], М14[ZrF8]. Stability of coordination compounds of halides is decreased in the series F- —Cl- —Br- — I-.
The summary of chemical properties of free elements of titanium subgroup is shown in the table below:
| Element | Conditions | Reagent | Product |
| Ti | Room temperature | HF(conc.) | TiF3 |
| HCl(conc.) | TiCl3 | ||
| Aqua regua | H2[TiCl6] | ||
| Heating | Air or oxygen | TiO2 | |
| Halogens | TiHal4 | ||
| S,N,C | TiS2, TiN, TiC | ||
| Molten NaOH | Titanates | ||
| Zr | Room temperature | HF(conc.) | H2[ZrF6] |
| Aqua regua | H2[ZrCl6] | ||
| Heating | Air or oxygen | ZrO2 | |
| Halogens | ZrHal4 | ||
| S,N,C | ZrS2, ZrN, ZrC | ||
| Molten NaOH | zirconates | ||
| Hf | Room temperature | HF(conc.) | H2[HfF6] |
| Aqua regua | H2[HfCl6] | ||
| Heating | Air or oxygen | HfO2 | |
| Halogens | HfHal4 | ||
| S,N,C | HfN, HfC | ||
| Molten KHF2 | K2[HfF6] |
Compounds with metallic type of bond. Hydrogen, carbon, nitrogen and some other nonmetals react with IVB group elements forming solid solutions of varying composition that are nonstoichiometric compounds (see also dioxygen reaction below).
Hydrides. At the beginning of reaction solid solutions of insertion of E2Н composition (mole fraction of Н2 is 33%) appear. The limiting composition will be EН2, when the temperature and hydrogen content are increased. The composition of the latter hydride can be varied from EH to EH2. It is a powder of gray or black colour.
Carbides and nitrides. Their preparation methods are illustrated by reactions below:
2Е + N2 = 2ЕN
2ЕО2 + 4С + N2 = 2ЕN + 4CO
Е + С = ЕС
ЕО2 + 3С = ЕС + 2СО
Chemically inert TiN reacts with steam and alkalis when subject to strong heating only:
2TiN + 4H2O = 2TiO2 + 2NH3 + H2
2TiN + 4KOH + 2H2O = 2K2TiO3 + 2NH3 + H2
Сarbides EС are metallike crystals that are good conductors, hard, and refractory (3000-4000 oC). They are chemically inert, although at high temperature they react with halogens, oxygen, nitrogen, for instance:
EС + 2Cl2 ® ECl4 + C
2TiC + N2 + H2 ® 2TiN + C2N2
Оxides and hydroxides (IV). Reactions of IVB subgroup elements with dioxygen include the stage of a solid solution of insertion type with molar fraction of oxygen up to 30%. The intermediates of this transformation are mostly nonstoichiometric. Let’s take titanium:
Ti + O2 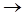 Ti6O
Ti6O 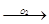 Ti3O
Ti3O 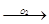 TiO
TiO 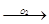 Ti2O3
Ti2O3 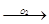 TiO2
TiO2
Oxides ЕО2 are refractory (tm TiO2 = 1870 oC, tm ZrO2 = 2850 oC, tпл HfO2 = 2900 oC), low active due to their polymeric structure. So, TiO2 represents 3D-polymer where each titanium atom is linked with 6 oxygenones, in turn, each oxygen is linked with 3 titaniumones. Oxides EO2 have no reaction with water, cold diluted acids (except HF) and alkalis. The long heating is the only method to realise reactions with acids. Displaying amphoteric properties, EO2 react with the molten alkalis and form salts.
Дата добавления: 2015-07-25; просмотров: 118 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Compounds | | | Hydroxides Е(ОН)4 are compounds of nonstoichiometric composition ЕО2.nH2O. Ortho-titanic (a-titanic) H4TiO4 (Ti(OH)4), and more inert meta-titanic(b-titanic) H2TiO3 are known. |