
|
Читайте также: |
PHYSICAL PROPERTIES
| Sc | Y | La | Ac | |
| density, g/сm3 | 3.0 | 4.5 | 6.2 | 10.1 |
| tm, oС | ||||
| tb, oС | ||||
| Rа, nm | 0.164 | 0.181 | 0.187 | 0.203 |
| R3+, nm | 0.083 | 0.097 | 0.104 | 0.111 |
| Stable nuclides | 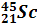 (100 %)
(100 %)
| 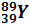 (100 %)
(100 %)
| 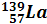 (99.91 %)
(99.91 %)
| - |
| Ionisation energy Е ® Е+, kJ∙mol-1 | ||||
| Е+ ® Е2+, kJ∙mol-1 | ||||
| Еo(Е3+ + 3е = Е), V | -2.08 | -2.37 | -2.52 | -2.60 |
Y, La, and Ac are silvery white metals. Sc has a yellowish colour. Sc аnd Y don`t corrode in normal conditions in dry air. La and Ac quickly become dark due to the thin oxide (hydroxide) film formation. Radioactive actinium glowes in the dark with a blue light.
Titanium subgroup
| Ti | Zr | Hf | |
| density, g/сm3 | 4.50 | 6.45 | 13.1 |
| tm, oС | |||
| tb, oС | |||
| Ti3+/Ti | Zr4+/Zr | Hf4+/Hf | |
| Еo(Е3+ + 3е = Е), V | -1.76 | -1.43 | -1.57 |
Titanium subgroup elements are silvery white malleable metals. Titanium belongs to light metals; Zr and Hf are heavy metals. All are refractory. Pure metals are easily subjected to a mechanical processing. On the other hand, they can be hard and brittle at absorbance of small amounts of О2 and N2 (for instance, after thermal processing). Therefore, their welding is only possible using argon-arc method. Ti, Zr, and Hf absorb Н2 significantly. They form phases of insertion.
Vanadium subgroup
| V | Nb | Ta | |
| Colour | Steel-gray | Light-gray | gray |
| Rа, nm | 0.134 | 0.146 | 0.146 |
| R5+, nm | 0.059 | 0.066 | 0.066 |
| Ionisation energy Е0 ® Е+, еV | 6.71 | 6.88 | 7.88 |
| density, g/сm3 | 5.96 | 8.57 | 16.6 |
| tm, oС | |||
| tb, oС |
V, Nb, and Ta are refractory metals with a volume-centred cubic unit cell. Their colour is usually silvery-gray and after oxidation of the surface it becomes dark. The chemical characteristics, properties of Ta and Nb are very similar since practically identical are their ionic and atomic radii. V, Nb, and Ta have high mechanical strength and malleability. When admixtures (O, N, C, B, H and other additives) are present they became brittle and hard because of compounds of varying composition with non-metals that are formed.
Дата добавления: 2015-07-25; просмотров: 68 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| які використовують для лікування дітей хворих на цукровий діабет | | | Vanadium Subgroup |