
|
Читайте также: |
MnO2 + C =
Mn + HCl =
Mn + HNO3 =
MnO2 + H2 =
MnSO4 + NaOH =
Mn(OH)2 + O2 + H2O =
Mn(OH)2 + Br2 + NaOH =
MnO2 + HCl =
MnO2 + H2SO4 =
MnO2 + KNO3 +
KOH 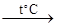
K2MnO4 + H2O =
K2MnO4 + Cl2 =
KMnO4 + Na2SO3 + H2SO4 =
KMnO4 + Na2SO3 + H2O =
KMnO4 + Na2SO3 + KOH =
KMnO4 + FeSO4 + H2SO4 =
KMnO4 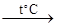
Re + O2 =
Re + HNO3 =
NH4ReO4 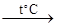
Re2O7 + H2O =
Experimental section
1. Materials and equipment: crystalline manganese (IV) oxide, potassium permanganate, sodium sulfite, solutions of hydrochloric acid, sulfuric acid, nitric acid, manganese (ІІ) sulfate, sodium hydroxide, ammonium persulfate, silver nitrate, chlorine water, hydrogen peroxide, concentrated hydrochloric acid, litmus paper, 100 mls beaker, test tubes, glass rods.
Дата добавления: 2015-07-25; просмотров: 49 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| The stability of compounds in the series Mn(VII) — Tc(VII) — Re(VII) is considerably increased but oxidising properties are decreased. | | | Chemical properties of manganese |