
|
Читайте также: |
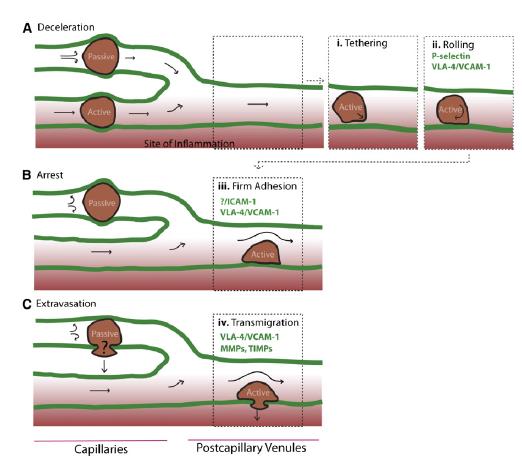
Homing refers to the ability of MSCs to migrate towards the damaged area, yet the mechanism of homing is not well understood. It is unclear if the MSCs actively home via leukocyte–like endothelial cell adhesion (via intravascular cellular adhesion, rolling and finally vascular extravasation, movement through stromal tissue and MSC engraftment) or become passively trapped in capillaries or microvessels (localization), instead of being arrested on endothelium by selectins and integrins. (Figure 10) (Karp and Teo., 2009)
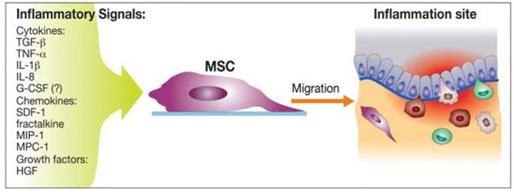
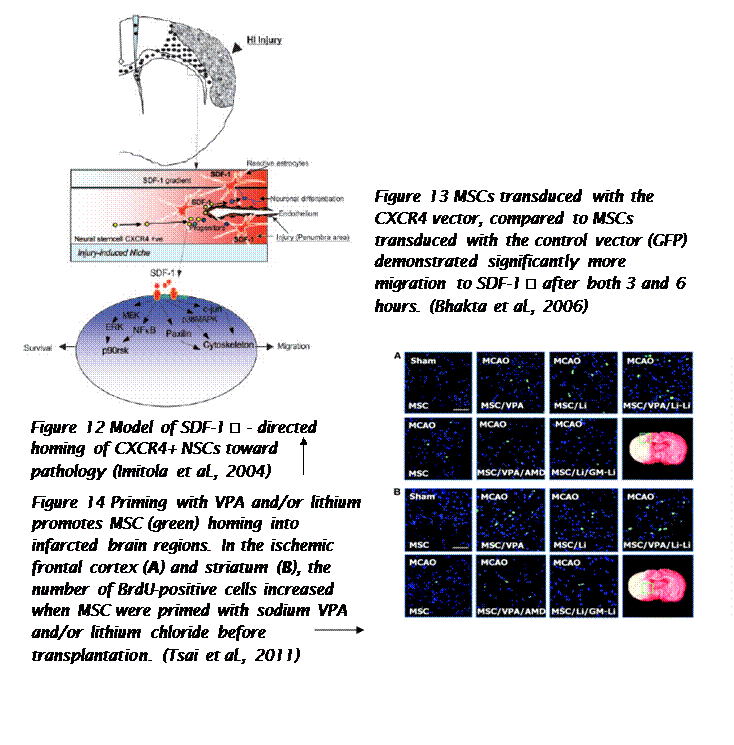
Biochemical messages from the damaged area, MSCs reception of them and chemotactic ability, influence MSC migration. It is challenging to determine the exact location of MSCs following infusion, as localization may occur, or MSCs may actually undergo transendothelial migration, leading to successful homing. (Karp and Teo., 2009) The conditions used to culture MSCs are very important, as they can have a significant impact on MSC function. The confluence of MSCs in culture, for example, affects migration potential, as increased culture confluence inhibits MSC homing by increasing the production of a natural matrix metalloproteinase (MMP) inhibitor, TIMP-3 (De Becker et al., 2007). Also, MSCs in culture can gain or lose some surface receptors, which influence their transendothelial migratory ability. Freshly isolated MSCs show improved homing capability, than culture-expanded MSCs. (Rombouts and Ploemacher, 2003). Tissue damage recruits MSCs into the inflicted sites, by cytokine and chemokine release and activation of adhesion ligands, so that MSCs can participate in tissue regeneration and modulation of the immune response. Examples of cytokines and chemokines produced by damaged tissues include: transforming growth factor (TGF-β), interleukin-1 (IL-1), tumour necrosis factor (TNF-α) and stromal derived factor (SDF-1). (Figure 11) MSC migration through type I collagen-rich stromal tissues, is dependent on MMP-2, MMP-9 and membrane-type-1 matrix metalloproteinase (MTI-MMP), which degrade components of the extracellular matrix, allowing for stem cell motility and later differentiation. Hepatocyte growth/scatter factor (HGF) is also produced by inflamed and damaged tissues, both human and mouse MSCs express a cognate receptor for HGF, c-Met. HGF induces MSC chemotaxis, yet inhibits proliferation. Imitola et al., (2004) show that neural stem cells (NSCs) migrate in vivo toward an infarcted area, where surviving local astrocytes, microglia and endothelium up-regulate the inflammatory chemoattractants, including SDF-1α, which direct the migration of NSCs towards the penubmra. Both exogenous and endogenous NSCs express the cognate receptor for SDF-1α, CXCR4, which allows them to migrate towards the source of chemokines, home to the ischaemically damaged region and produce anti-inflammatory and anti-scarring molecules. (Figure 12) Bhakta et al., (2006) state that most MSCs do not possess CXCR4, however, to optimize their migration and survival, CXCR4 gene can be expressed using retroviral transduction. In this study, MSCs were isolated from healthy volunteers, cultured and transduced with either a CXCR4 containing retroviral vector and green fluorescent protein (GFP) or only GFP - control vector. As expected, MSCs transduced with CXCR4 migrated significantly more toward SDF-1α. (Figure 13) Findings by Cheng et al., (2008) also support this statement. A study by Tsai et al., (2011) has also found that priming MSCs with VPA or lithium increased homing to the cerebral infarcted regions via CXCR4 and MMP-9 upregulation, and copriming with VPA and lithium further enhanced this effect. MSC were labelled with BrdU before transplantation. Homing MSCs were detected using immunohistochemistry. (Figure 14) Therefore, both SDF-1α and MMP-9 expression appear to be essential for the homing ability of stem cells.
|
|
|
|
statement.
 | |||
|
|
Such variability of MSC properties emphasizes the importance of comprehensive characterization of MSCs within each study. It is especially important to have an accurate assessment of MSC properties prior to injection or implantation of MSCs into the highly complex and varying microenvironments that exist within the body.
Discussion
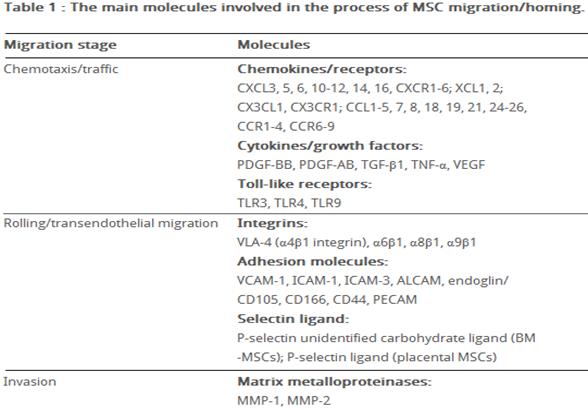
Previously, several groups have studied the expression of chemokine receptors on human MSCs derived from bone marrow. Wynn et al., [56] evaluated the expression of CXCR4 on hMSCs isolated from bone marrow (hBMMSCs), and showed that the receptor is present on the cell surface membrane of less than 1% of the cells; although they found, that 83% -98% of the hBM-MSCs expressed intracellular CXCR4 at high level. Other studies have shown functional expression of CXCR4 at the surface of BM-MSCs [57]; expression of CCR1, CCR7, CXCR4, CXCR6, and CX3CR1 was shown on individual cells (2-25%) [52]. Honczarenko M. et al., [58] performed flow cytometric analysis of the expression of CC chemokine receptors (except for CCR10), CXC receptors and CX3CR1. They found, that 43%–70% of the hBM-MSCs expressed functional (as determined by chemotaxis assay) CCR1, CCR7, CCR9, CXCR4, CXCR5 and CXCR6. Another group showed expression of CCR2, CCR8, CXCR1, CXCR2, and CXCR3 in BM-MSCs by RT-PCR and immunohistochemistry [59]. Ponte et al., [60] demonstrated that hBM-MSCs expressed CCR2, CCR3, CCR4, and CXCR4, and found that TNF increased the expression of CCR2, CCR3 and CCR4, but not CXCR4.
Despite contradictory data obtained in different studies concerning expression of several chemokine receptors, it is obvious that MSCs express a great variety of these receptors. Such contradictions may be due to several factors: the heterogeneity of ex vivo expanded MSCs, which is a feature of these cells, the differences in the methods of MSCs isolation and cultivation and the number of passages that have undergone cells before analysis. In this context, the number of passages is particularly important in the analysis of chemokine receptor expression. Thus, ex vivo cultured MSCs, which were initially positive for CCR1, CCR7, CCR9, CXCR4, CXCR5, and CXCR6, at passage 12-16 loss of their surface chemokine receptor expression, which was accompanied by a lack of chemotactic response to chemokines, and by further decline in the expression of adhesion molecules, including ICAM-1, ICAM-2 and VCAM-1 [58]. In addition to the long-term cultivation of MSCs and to cultural conditions, changes in the expression pattern of surface receptors dependent on confluency of cells, site of isolation and the environment in the process of incubation (normoxic or hypoxic conditions) [
Conclusion
|
References
1) National Institutes of Health, U.S. Department of Health and Human Services. (2002). Stem Cell basics. Available: http://stemcells.nih.gov/info/basics/pages/basics1.aspx. Last accessed: 15/04/2014.
2) Kishk N et al. (2010). Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 24 (8), p. 702-8.
3) Liang Y et al. (2013). The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chinese Journal of Cancer, 32 (4), p. 205-212.
4) Yamanaka S. and Blau H., (2010). Nuclear reprogramming to a pluripotent state by three approaches. Nature, 465, p. 704–712
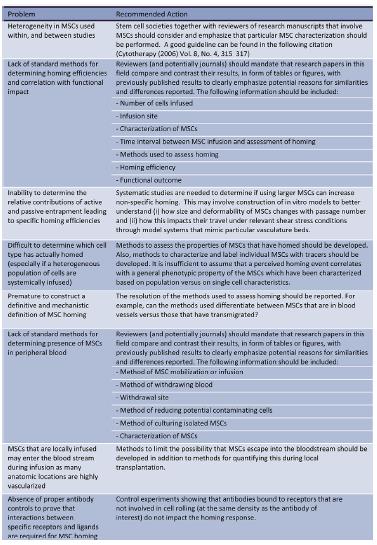
5) Dominici M. et al., (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8 (4), p. 315-317.
6) Colledge N., Walker B., Ralston S. (2010). Davidson's Principles and Practice of Medicine. 21st ed. Elsevier. p. 1181-1182.
7) Bhakta S. Et al., (2005) The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovascular Revascularization Medicine. 7 (1), p. 17-24.
8) Chen L., et al. (2008). Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE. 3, (4)
9) Tsai L. et al.,. (2011). Mesenchymal Stem Cells Primed With Valproate and Lithium Robustly Migrate to Infarcted Regions and Facilitate Recovery in a Stroke Model. Stroke. 42, p. 2932-2939
10) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3185169/
Дата добавления: 2015-08-18; просмотров: 58 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| MSCs for treatment of stroke | | | Introduction |