
|
Читайте также: |
These are of two general kinds: octahedral, pink coordination compounds and tetrahedral: blue complexes. If cobalt(II) chloride is dissolved in aqueous solution, the predominant species is the hexaaquo-ion [Co(H2O)6]2+ (pink). If this solution is heated, it becomes blue, and the same effect is observed if chloride ion is added in excess. This colour change is associated with the transformation
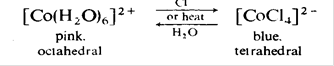
but ions intermediate between these two species can also exist in the solution. None of these species can be oxidised to cobalt(III) in aqueous solution; but if ammonia is added to the pink solution containing the hexaaquo-ion, the water ligands are displaced by ammonia and the hexammino-ion [Co(NH3)6]2+ is formed; this is easily oxidised to the +3 state. A large number of other cobalt(II) complexes, cationic, neutral and anionic are known.
Historically, this was observed as early as 1798. Tassaert observed that an ammoniacal solution of a cobalt(II) salt changed colour at exposure to air, and some years later it was shown that, if cobalt(II) chloride was oxidized in the presence of ammonia, the yellow product had the formula CoCl3. 6NH3, a formula which posed a valency problem to the chemists of that time. Alfred Werner, in the period 1890—1913 (he was awarded the Nobel Prize for chemistry in 1913), was primarily concerned with explanation the nature of CoCl3 6NH3 and similar compounds; his investigations (carried out in the absence of the structural methods available to us today) showed conclusively that the compound was a complex [Co(NH3)6]Cl3, hexamminocobalt(III) chloride, and Werner pioneered the study of coordination compounds.
Cobalt(III) contains six 3d electrons; in the presence of six appropriate ligands, arranged octahedrally, a large splitting of the d orbitals occurs, and all these electrons are paired in a more stable energy level. Such an arrangement is stable with respect to oxidation or reduction. "Appropriate" ligands are those containing a nitrogen donor atom, for example ammonia NH3, cyanide CN- and nitro — NO2-, and cobalt has a strong affinity for all these. Thus if cobalt(II) chloride is oxidized by air in the presence of ammonia, with ammonium chloride added to provide the required anion, the orange hexamminocobalt(III) chloride is precipitated:
4[Co(H2O)6]Cl2 + 4NH4C1 + 20NH3 + O2 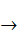 4[Co(NH3)6]Cl3 + 26H2O.
4[Co(NH3)6]Cl3 + 26H2O.
For this reaction, charcoal is a catalyst; if this is omitted and hydrogen peroxide is used as the oxidant, a red aquopentamminocobalt(III) chloride, [Co(NH3)5H2O]Cl3, is formed and treatment of this with concentrated hydrochloric acid gives the red chloropentammino-cobalt(III) chloride, [Co(NH3)5Cl]Cl2. In these latter two compounds, one ammonia ligand is replaced by one water molecule or one chloride ion; it is a peculiarity of cobalt that these replacements are so easy and the pure products so readily isolated. In the examples quoted, the complex cobalt(III) state is easily obtained by oxidation of cobalt(II) in the presence of ammonia, since
[Co(NH3)6]3+(aq) +e- 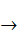 [Co(NH3)6]2 + (aq), E0 = +0.1 V.
[Co(NH3)6]2 + (aq), E0 = +0.1 V.
Cobalt(II) is also easily oxidised in the presence of the nitrite ion NO 2 as ligand. Thus, if excess sodium nitrite is added to a cobalt(II) salt in presence of acetic acid (a strong acid would decompose the nitrite), the following reaction occurs:
Co2+(aq) + 7NO2- + 2H+ = NO + H2O + [Co(NO2)6]3–
Co(ІІІ):
4CoCl2 + 20NH3 + 4NH4Cl + O2 = [Co(NH3)6]Cl3 + 2H2O,
4Co(NO3)2 + 24NaNO2 + 4CH3COOH + O2 = 4Na3[Co(NO2)6] + 8NaNO3 + 4CH3COONa + 2H2O
Here, effectively, the Co2+(aq) is being oxidised by the nitrite ion and the latter (in excess) is simultaneously acting as a ligand to form the hexamtrocobaltate(III) anion. In the presence of cyanide ion CN. cobalt(II) salts actually reduce water to hydrogen since
 [Co(CN)6]3–(aq) + e–
[Co(CN)6]3–(aq) + e–  [Co(CN)5(H2O)]3+(aq) + CN‑; E0= –0.8V.
[Co(CN)5(H2O)]3+(aq) + CN‑; E0= –0.8V.
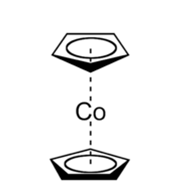
Cobaltocene, Co(C5H5)2, is known as bis(cyclopentadienyl)cobalt(II). Co(C5H5)2 belongs to a group of organometallic compounds called metallocenes that consist of a metal atom sandwiched between two cyclopentadienyl (Cp) rings.
Дата добавления: 2015-07-25; просмотров: 86 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| COMPLEXES OF IRON | | | COORDINATION COMPOUNDS OF NICKEL |