
Читайте также:
|
Iron. The extraction of iron began several thousand years ago, and it is still the most important metal in everyday life because of its abundance and cheapness, and its ability to be cast, drawn and forged for a variety of uses.
The process of extraction requires first smelting and then refining.
In smelting, iron ore (usually an oxide) is mixed with coke and limestone and heated, and hot air (often enriched with oxygen) is blown in from beneath (in a blast furnace). At the lower, hotter part of the furnace, carbon monoxide is produced and this is the essential reducing agent.
The occurring reduction reactions may be presented for simplicity as:
3CO + Fe2O3 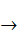 2Fe + 3CO2, (1)
2Fe + 3CO2, (1)
Fe2O3 + CO 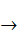 2FeO + CO2, (2)
2FeO + CO2, (2)
FeO + C - 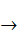 Fe + CO. (3)
Fe + CO. (3)
Reaction (1) is exothermic and reversible, and begins at about 700 K; by Le Chateliers Principle, more iron is produced higher up the furnace (cooler) than below (hotter). In the hotter region (around 900 K), reaction (2) occurs irreversibly, and the iron(II) oxide formed is reduced by the coke (reaction (3)) further down.
The limestone forms calcium oxide which fuses with admixtures of alumosilicates in the ore to give a slag of calcium silicate; it floats on the molten iron (which falls to the bottom of the furnace) and can be run off at intervals. The iron is run off and solidified as "pigs'—boat-shaped pieces about 40 cm long.
Pig-iron or cast iron contains impurities, chiefly carbon (up to 5%) free or combined as iron carbides. These impurities, some of which form interstitial compounds with the iron, make it hard and brittle, and it melts fairly sharply at temperatures between 1400 and 1500 K; pure iron becomes soft before it melts (at 1812 K). Hence cast iron cannot be forged or welded.


When iron is refined, the process is essentially one of melting iron in presence of materials which will react with the impurities— for example air (or oxygen) to remove chiefly carbon, and calcium oxide (added as carbonate) to remove phosphorus.
There are a variety of refining processes, each depending on the composition of the initial iron and the sort of iron or steel destined as the end product. Steels have a carbon content of 0.1-1.5%, and addition of other transition metals imparts certain properties (for example a little manganese, elasticity and high tensile strength; more manganese, great hardness; chromium, resistance to chemical attack, as in stainless steel; nickel, a reduced expansion; tungsten and vanadium, hardness retained at high temperatures).
Pure iron is produced in various ways. The most important of them are the thermal decomposition of iron pentacarbonyl, the electrolysis of an iron(II)-containing aqueous solution, and reduction of iron(II) oxide with hydrogen.
Cobalt. The metal itself has been produced on an industrial scale only during the twentieth century. Extraction is carried out by a process essentially similar to that used for iron, but is complicated because of the need to remove arsenic and other metals.
Nickel. The metal is obtained by heating with sulfur compounds to give the sulfide, which is roasted to form the oxide; the latter may be reduced directly by heating with coke or dissolved to give a solution containing nickel(II) from which the nickel can be deposited electrolytically. The metal obtained by reduction can be purified by the Mond process, in which it is heated to 320 K with carbon monoxide to give the pure, volatile tetracarbonyl Ni(CO)4; the latter when heated to 500 K gives the pure metal and carbon monoxide is recovered:
Ni + 4CO 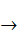 [Ni(CO)4].
[Ni(CO)4].
Uses
Iron. Iron(II) sulfate is used to combat plant pests, in the production of ink and mineral pigments, and in the dyeing of fabrics. Iron(III) chloride is used as a coagulant in water purification, and as a catalyst in organic syntheses and in the textile industry.
The insoluble brown copper(II) salt Cu2[Fe(CN)6] once much used as a semipermeable membrane in osmotic pressure determinations.

The Iron Bridge over the River Severn at Coalbrookdale, England
Cobalt. Cobalt is used in alloys for special purposes. Cobalt compounds have been in use for centuries, notably as pigments ("cobalt blue") in glass and porcelain (a double silicate of cobalt and potassium);
Nickel. Nickel is a moderately lustrous, silvery metal, and is extensively used in alloys (for example coinage, stainless steel) and for plating where a durable resistant surface is required. It is also used as an industrial catalyst, for example in the hydrogenation of unsaturated organic compounds.
Дата добавления: 2015-07-25; просмотров: 121 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Iron triad trends | | | Free elements |