
|
Читайте также: |
We conducted a series of laboratory experiments by changing the composition of soil-soil, the dose "supergumusa" and nitrogen fertilizer. Experiments were carried out in plastic columns with a diameter 5.0 cm and length-tion 100.In the early to check the nitrogen content and pH as in "supergumuse" and in the soil itself. To do this, the bottom of the column put a cotton swab. Then in the first column (№ 1) poured "supergumus" and the second (№ 2) soil-clean soil (heavy clay loam). In both columns poured on top disteli-dated water (to eliminate the effect of nitrogen compounds, which are contained in the water). The eluate was collected in 50 ml of which quantitatively determined the pH, NO-3 (pH meter on), NO-2 - Griess reagent and NH+4 the Nessler reagent. Collected in 10 fractions. The results are shown in Table 1. The table shows that "supergumus" product is acidic with a pH of 3.5. Virtually no nitrate (NO-3) and nitrite (NO-2). Ammonium (NH+4) is contained in a decent amount decreased for two days with 12.0 mg / l to 4.0 mg / liter. Ammonium is pretty easy to consume plants, including cotton. In addition, it is more difficult to migrate the profile area Aera-tion, subjected to exchange reactions. In the soil-soil (column 2), mainly in small quantities (10-42 mg / l) contained nitrate and nitrite (0,4-9,0 mg / l), ammonium is small (0,7-2,1 mg / l) and the medium is slightly alkaline (pH 8,10-8,95).
Table 1 shows that the "supergumus" product is acidic with a pH of 3.5. There is practically no nitrate (NO-3), and nitrite (NO-2). Ammonium (NH+4) is contained in decent quantities, fell in two days with 12.0 mg / l to 4.0 mg / liter.
In the soil-soil (column number 2) in small amounts (10-42 mg / l) is contained nitrate and nitrite (0,4-9,0), very little ammonium (0,7-2,1 mg / l.) Wednesday is slightly alkaline (pH = 8,10-8,95). The results are shown in Table 2. Since our objective of studying the migration of nitrate in the eluate was further subjected to chemical analysis, only NO-3, and pH.
| I- column (superhumus) mg/l | II-column (soil) mg/l | ||||||||
| рН | 
| 
| 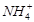
| рН | 
| 
| 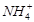
| ||
| Sample №1-1 | 3,50 | сл. | 0,01 | Sample №2-1 | 8,10 | 2,1 | |||
| Sample№1-2 | 3,40 | сл. | 0,01 | Sample №2-2 | 8,25 | 2,1 | |||
| Sample №1-3 | 3,40 | сл. | 0,01 | Sample №2-3 | 8,30 | 1,5 | |||
| Sample №1-4 | 3,50 | сл. | 0,01 | Sample №2-4 | 8,40 | 1,2 | |||
| Sample №1-5 | 3,50 | сл. | нет | Sample №2-5 | 8,70 | 1,2 | |||
| Sample №1-6 | 3,45 | сл. | 0,02 | Sample №2-6 | 8,90 | 1,0 | |||
| Sample №1-7 | 3,60 | сл. | сл. | Sample№2-7 | 8,85 | 0,6 | 0,7 | ||
| Sample №1-8 | 3,60 | сл. | сл. | Sample №2-8 | 8,90 | 0,6 | 0,7 | ||
| Sample №1-9 | 3,50 | сл. | сл. | Sample №2-9 | 8,95 | 0,4 | 0,7 | ||
| Sample№1-10 | 3,50 | сл. | сл. | Sample №2-10 | - | - | - | - |
Table 1.The content of nitrogen in the soil supergumuse
| I- column (superhumus) mg/l | II-column (control) mg/l | ||||
| filtrate | рН | 
| filtrate | рН | 
|
| №1 | 7,30 | №1 | 7,40 | ||
| №2 | 8,30 | №2 | 8,00 | ||
| №3 | 8,40 | №3 | 6,65 | ||
| №4 | 8,70 | №4 | 8,30 | ||
| №5 | 9,00 | №5 | 8,20 |
Table 2. Dynamics of nitrogen and pH
The table shows that from the column with supergumusom nitrate (NO-3), washed in smaller numbers than in the control variant. In the first leachate nitrate was reduced by 55%, the second at 40%, the third faction turned out abnormal, so it does not take into account, the fourth fraction contained 12.5% less. Nitrate (NO-2) in supergumuse was not so washed out in both columns is more evenly from ammonium nitrate. Nitrite virtually contained in the fertilizer, but as we saw above, is formed as an intermediate product of nitrification. Several countries in the pH of filtrate. Above, we have determined that the pH supergumusa 3.5. But in column number 1 since the publication of the filtrate slightly alkaline pH, from pH 7.30 to 9.00. The mechanism of this process is difficult to explain. In the next series, we changed the amount of applied nitrogen fertilizer and supergumusa. In column number 1 (completed in the first experiment, heavy loam) have 10g and 1.0 g supergumusa ammonium nitrate. In column number 2 only 1.0 grams of fertilizers. Experiments began November 15, 2006. and after charging the column in 1130 surged by 200 ml of water distelirovannoy. No infiltration, and in 1500 added another 200 ml of water. The first portions of the filtrate (50 ml) were the next day on November 16. Since our goal is to develop a method of aeration zone and groundwater from nitrates, but in future we were subjected to chemical analysis of samples only (NO-3) and pH. We must follow the acidity of the filtrate, as supergumus, as we defined above, the acid.
Subsequently, all experiments were positive, showing that nitrogen retards supergumus soedineniyab and after a hard metal. And also a fruitful harvest. The experiments were carried out in field and laboratory conditions.
Findings
1. "Supegumus" has a positive effect on the sorption of nitrates, essentially keeping them in soils during irrigation.
2. "Supergumus" no negative effect on the pH of water infiltration.
References:
1. Zarubkin G.P., Dmitriev M.T., Prihodko E.M., Mishihin V.M. «Hygiene and Sanitation» 1984,7, 49-52.
2. Novikov Yu.V., Okladnikov N.I., Sayfutginov M.M., Andreev M.A. «Hygiene and Sanitation» 1985, 8, 58-62.
3. Environmental problems of fertilizer application. "Science"1984.
4. Pushkarev M.M., Chegibkina L.A. Chemistry in Agriculture 1980, 10, 6-9.
5. Yarvan M.E.Chemistry in Agriculture. 1982 №3.
6. Hodjaev V.G. Agrochemistry, 1983, 6, 11-16.
7. Hodjaev V.G., Jumanov H.N., Samoylenko V.G. «The use of nitrification inhibitors to increase the efficiency of nitrogen fertilizer. "Proceedings of All-Union Conference (Samarkand), Moscow 1990 year. 72-74.
8. Hodjaev V.G. «Hygiene and Sanitation» 1987, 11, 68-69.
Дата добавления: 2015-10-29; просмотров: 115 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Investigation of influence "supergumusa" on migration nitrate in the profile of the aeration zone | | | Speciation of gold in peat polluted by acid main drainage of Ursk tailing pit |