
Читайте также:
|
Yudin D.S., Pokhilenko L.N., Alifirova T.A., Travin A.V., Zhimulev E.I., Alekseev D.V., Ponomarchuk A.V.
V.S. Sobolev Institute of Geology and Mineralogy SB RAS, Novosibirsk, Russia
dsyudin@gmail.com
Temperature is the main factor affecting on the mobility of argon in the mineral essential for the interpretation of 40Ar/39Ar data. The effect of pressure is usually considered to be insignificant. But for object of thermal reconstruction having undergone high pressures (e.g. from deep-seated xenoliths, subduction zones, or zones of intense deformation) it is necessary to take into account the pressure. Unfortunately, the available published data for quantifying the effects of pressure on the mobility of radiogenic argon is limited.
We have conducted a series of laboratory experiments to assess the influence of high temperatures and pressures (with conditions close to that of transporting within kimberlitic melt) to the stability of 40Ar/39Ar isotopic system in mica. Experiments were carried out using high-pressure high-temperature split-sphere multi-anvil apparatus (BARS) at IGM SB RAS (Novosibirsk).
Biotite MSA-11 prepared by VIMS in 1988 as a K/Ar standard was used as the sample. The biotite MSA-11 with a clear plateau at the age spectrum (Fig.) has been certified by us as 40Ar/39Ar monitor using international standard samples of muscovite Bern 4m, biotite LP-6 [1]. The mean value of calibration results equal to 311.0 ± 1.5 million years has been adopted as an integral age of the biotite MSA-11. During experiments the periclase was used as a buffer.
Six laboratory experiments were conducted:
1. without heating, 40 kbar, (4-27-11);
2. 1200ºС, 10 kbar, 2 hours (4-47-11);
3. 1150ºС, 10 kbar, ~1 minute (4-44-11);
4. 1200ºС, 40 kbar, 10 minutes (4-28-11);
5. 1200ºС, 40 kbar, 2,5 hours (4-31-11);
6. 1200ºС, 20 kbar, 2 hours (4-41-11).
The initial sample and the samples obtained after laboratory experiments were analyzed using TESCAN scanning electron microscope (IGM SB RAS).
МSА-11 The biotite is of plate morphlogy. The composition of the mica is almost homogeneous and shows no differences depending on the area of analysis. The Fe# (= 100Fe/Fe + Mg) of biotite is 63.0-65.0.
4-27-11 (without heating, 40 kbar) The plates of biotite are strongly curved. There are thin layers of muscovite. The compositions of the micas are almost homogeneous and show no differences depending on the area of analysis. The Fe# (= 100Fe/Fe + Mg) of biotite are equal to 63.0-65.0.
4-47-11 (1200ºС, 10 kbar, 2 hours) Biotite from the sample is in the form of well-cut plates up to 600 microns in length, usually 150-200 microns. There are also grains of periclase and olivine of varying Fe# (10.7 to 41.2). A K-bearing alumosilicate glass (with K2O up to 20.5 wt %) is between the phlogopite plates. Biotites show essential compositional variations from one to another, especially at Fe# (35.2 to 60.8). Extra Fe-rich compositions (Fe # is up to 86.8-96.4) are typical for the rims of the biotite grains. The TiO2 content is cover a range of 1.24-3.54 wt %. Compositional variations show zonal and spotted distribution within biotite grains.
4-44-11 (1150ºС, 10 kbar, ~1 minute) Fine-grained aggregates of biotite are up to 100 microns in length, more often 20 to 60 microns. There is a high-silicon K-phase in the interstices between the large biotite plates. These plates often form intergrowths with euhedral grains of spinel (hercynite). Biotite varies in composition (Fe# 50.2-63.7, and TiO2 0.95-3.16 wt %).
4-28-11 (1200ºС, 40 kbar, 10 minutes) It is a fine-grained aggregate of biotite and to a lesser extent garnet (almandine). The length of the biotite plates are up to 100 microns, the garnet grains are usually 10-50 microns in diameter. The distinct grains of olivine were observed. A site with high Fe content in minerals was revealed at the sample. Fe content in this site increases markedly in biotite (Fe# 84.4), and garnet (Fe# 85.7-88.0) compare to the composition of these minerals beyond Fe-enriched zone (Fe# of biotite and garnet 53.8-64.6 and 72.7, respectively).
4-31-11 (1200ºС, 40 kbar, 2,5 hours) It is a fine-grained aggregate of biotite, corundum, spinel (hercynite), olivine, and ilmenite. The length of biotite plates rarely exceeds 100 microns, occasionally reaching 200-250 microns. The Fe content of the biotite at the marginal parts of the sample is lower (Fe# 23.0-31.9) than at the center of the sample (Fe# 47.3-65.1). Biotite compositions slightly range in TiO2 content (1.4-2.49 wt %). The K-bearing phase (up to 28.8 wt % of K2O) in the interstices is observed.
4-41-11 (1200ºС, 20 kbar, 2 hours) It is a fine-grained aggregate of biotite, spinel (hercynite), olivine, and high-potassium glass (K2O is up to 19.3 wt %). Significant variations of Fe# (23.5-88.0) and TiO2 content (0.9-2.98 wt %) in biotites were revealed. The biotite with low Fe content is usually characterized by small content of TiO2.
The studies of biotite MSA-11 conducted before and after the experiment generally show preservation of its chemical composition. The size of the plates of mica decreases up to about 0.05 mm under pressure. The number of thin, newly formed during the experiment lamellae of biotite with slightly different composition, and size less than 0.05 mm is small when compared to the total contention of biotite.
The 40Ar/39Ar step-heating studies of the samples obtained after laboratory experiments were carried out to estimate the distribution of the argon.

Fig. The age spectra reflecting the distribution of argon both in the grain of standard MSA-11 and of mica subjected to laboratory experiments.
The experiments showed that the exposure of high temperature and pressure had caused the significant loss of radiogenic argon in standard sample MSA-11. The 99.3% loss of radiogenic argon occurred as a result of residence of the biotite standard at 1200°C and pressure of 20 kbar during 2 hours. Given that pressure was 2 times enhanced, the loss of radiogenic argon increased to 95.9%.
Based on preliminary results it can be concluded that under deep conditions at high temperatures (about 1200ºC) and pressures (20-40 kbar), decreasing of argon diffusivity in micas occurs with an increasing of pressure. The results obtained confirm the suggestion that despite high temperatures K / Ar mineral systems could occasionally be "locked" at sufficiently high pressures [2].
This study was supported by RFBR (projects № 11-05-00144, 11-05-00758) and Russian President Grant МК-3495.2012.5.
References:
1. Baksi A.K., Archibald D.A., Farrar E. Intercalibration of 40Ar/39Ar dating standards // Chemical Geology. 1996. V. 129. P. 307-324.
2. Baxter E. F. Diffusion of Noble Gases in Minerals // Reviews in Mineralogy and Geochemistry 2010. V. 72. № 1. P. 509-557.
MINERALOGY
New data on the crystal structure of metavivianite, Fe2+Fe3+2(PO4)2(OH) 2 ·6H2O
Aksenov S.M.1, Rastsvetaeva R.K. 1, Chukanov N.V.2
1Institute of Crystallography RAS, Moscow, Russia
2Institute of Problems of Chemical Physics RAS, Chernogolovka, Russia
rast@ns.crys.ras.ru
Metavivianite was first described with the formula Fe3(PO4)2·8H2O and was considered as a dimorph of vivianite, isostructural with symplesite [1]. However this description is incomplete and corresponds to a heterogeneous material. In addition, no separate analyses were reported for Fe2+ and Fe3+, as well as for H2O. In the cited work, the crystal structure of metavivianite was not investigated; the analogy with symplesite was supposed basing on the similarity of X-ray powder-diffraction data. Subsequent investigations [2, 3] have shown that metavivianite contains Fe3+ and is not dimorphous with vivianite. However the crystal structure of metavivianite was solved with a high R factor of 13.3%, and the formula Fe2+3-xFe3+x(PO4)2(OH) x ·(8– x)H2O suggested for metavivianite in the cited works does not reflect crystal-chemical features of this mineral, in particular, the distribution of Fe2+ and Fe3+ between two independent sites.
In this work, crystal structure investigation of metavivianite has been carried on a homogeneous sample from the Boa Vista pegmatite, Brazil. The chemical composition is (electron microprobe combined with Mössbauer data, mean of 5 analyses, wt.%): MgO 0.70, MnO 0.92, FeO 17.98, Fe2O3 26.60, P2O5 28.62, H2O (by gas chromatography) 26.5; total 101.32. The mineral is triclinic, a = 4.629(1), b = 7.989(1), c = 9.321(2) Å, a = 108.59(2), b = 97.34(1), g = 95.96(1)°, V = 320.18(11) Å3, Z = 1. Single-crystal X-ray data were collected using diffractometer Xcalibur Oxford Diffraction with CCD-detector. The crystal structure of metavivianite was solved by “charge flipping” method and refined in space group P  with corresponding R -index 6.0% for 1350 | F obs| > 3σ F. The structure consists of heteropolyhedral layers along diagonal bc. Each layer contains pairs of edge-sharing octahedra with the composition [Fe3+2O4(H2O,OH)4] and isolated octahedra [Fe2+O2(H2O)4] combined by [PO4]-tetrahedra (Fig.). The layers are linked by hydrogen bonds formed by water molecules and OH groups.
with corresponding R -index 6.0% for 1350 | F obs| > 3σ F. The structure consists of heteropolyhedral layers along diagonal bc. Each layer contains pairs of edge-sharing octahedra with the composition [Fe3+2O4(H2O,OH)4] and isolated octahedra [Fe2+O2(H2O)4] combined by [PO4]-tetrahedra (Fig.). The layers are linked by hydrogen bonds formed by water molecules and OH groups.
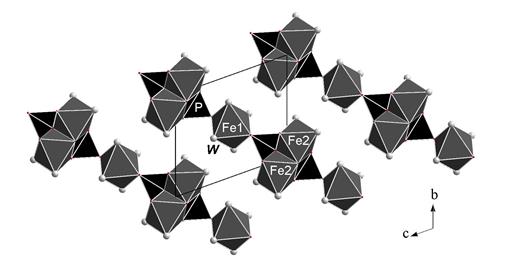
Fig. The crystal structure of metavivianite, the cb projection.
| Site | O1 | O2 | O3 | O4 | W 1 | W 2 | W 3 | W 4 | Vi |
| Fe1 | 0.40(x2)→ 0.40↓ | 0.29(x2)→ 0.29↓ | 0.36(x2)→ 0.36↓ | 2.1 | |||||
| Fe2 | 0.43 | 0.50 | (0.41+0.37)↓→ | 0.51 | 0.41 | 2.63 | |||
| P | 1.21 | 1.23 | 1.28 | 1.19 | 4.91 | ||||
| Vi* | 1.64+ (0.42) | 1.73+ (0.18) | 1.68+ (0.22) | 1.97 | 0.51+ (0.2) | 0.29+ (0.18) | 0.41+ (0.18) | 0.36+ (0.2) |
Table. Bond valence calculations for metavivianite
*Bond valences for anions are supplemented by contributions (in brackets) from hydrogen atoms of water molecules.
The distribution of cations between octahedral sites was made taking into account the ratio Fe2+: Fe3+ = 43:57 (obtained from the Mössbauer spectrum), the values of interatomic distances and results of bond valence calculations (Table). Although the H-atoms were not localized, we took into account the distances between O atoms of water molecules that are equal to ~2.75 Ǻ. The values of bond valence corresponding to these distances are ~ 0.2 v.u. With the account of these contributions we assume that W 1 and W 3 can partly be replaced by OH groups.
The refined crystal-chemical formula of metavivianite (Z =1) is [(Fe2+0.93Mn0.07)(H2O)4] [(Fe3+0.81Fe2+0.11Mg0.08)2(H2O,OH)4] [PO4]2. Bond valence calculation for Fe3+ in Fe2 position gives low sum, that corresponds to the presence of bivalent elements (Table). The simplified crystal-chemical formula of metavivianite is Fe2+(Fe3+,Fe2+)2(PO4)2(OH,H2O)2·6H2O. Bond valence calculations for metavivianite show that Fe2+ and Fe3+ are predominant components in the sites Fe1 and Fe2 respectively in opposite to the sample investigated by Dormann et al. (1982), in which both sites are predominantly occupied by Fe3+ Consequently, the idealized (end-member) formula of this mineral is Fe2+Fe3+2(PO4)2(OH) 2 ·6H2O. In other words, metavivianite is dimorphous with ferrostrunzite. As distinct from ferrostrunzite, metavivianite is a transformational mineral species formed as a result of natural oxidation of vivianite when the content of Fe3+ exceeds 1.4 pfu.
This work was financially supported by Russian Foundation for Basic Research (grant No. 10-05-00092-a). The authors thank R. Scholz for the sample of metavivianite.
References:
1. Ritz, C., Essene, E.J. and Peacor, D.R. (1974) Metavivianite, Fe3(PO4)2·8H2O, a new mineral. American Mineralogist, 59, P. 896 – 899.
2. Dormann, J., Gaspérin, M. and Poullen, J.-F. (1982) Étude structurale de la séquence d'oxydation de la vivianite Fe3(PO4)2·8(H2O). Bulletin de
Minéralogie, 105, P.147 – 160 (in French).
3. Rodgers, K.A. (1986) Metavivianite and kerchenite: a review. Mineralogical Magazine, 50, P. 687 – 691.
Дата добавления: 2015-10-29; просмотров: 176 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Experimental study of silver-palladium sellenides | | | Kyanite eclogite xenolith from Udachnaya pipe: whether there was coesite in the rock |