
|
Читайте также: |
 |
• Faster reaction but it reaches a saturation point when all the enzyme molecules are occupied by substrates
• If you alter the concentration of the enzyme then Vmax will change too.
pH: Most enzymes work best at a pH close to neutral (pH7), but there are some exceptions. Pepsin, an enzyme found in the stomach, has an optimum pH of 2.
• 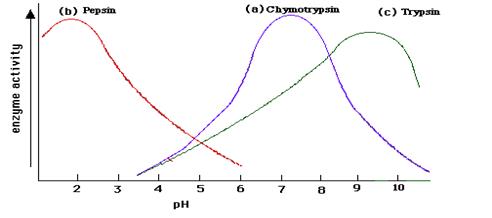
• Extreme pH levels will produce denaturation
• The structure of the enzyme is changed
• The active site is distorted and the substrate molecules will no longer fit in it
• At pH values slightly different from the enzyme’s optimum value, small changes in the charges of the enzyme and it’s substrate molecules will occur
• This change in ionisation will affect the binding of the substrate with the active site.
Temperature: 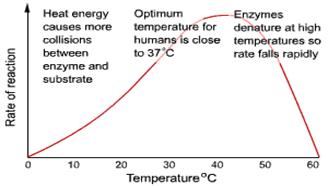
• Q10 (the temperature coefficient) = the increase in reaction rate with a 10°C rise in temperature.
• For chemical reactions the Q10 = 2 to 3
(the rate of the reaction doubles or triples with every 10°C rise in temperature)
• Enzyme-controlled reactions follow this rule as they are chemical reactions
• BUT at high temperatures proteins denature
• The optimum temperature for an enzyme controlled reaction will be a balance between the Q10 and denaturation.
• For most enzymes the optimum temperature is about 30°C
• Many are a lot lower, cold water fish will die at 30°C because their enzymes denature
• A few bacteria have enzymes that can withstand at very high temperatures up to 100°C
• Most enzymes however are fully denatured at 70°C
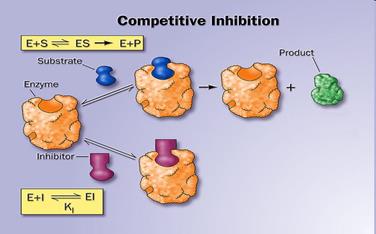
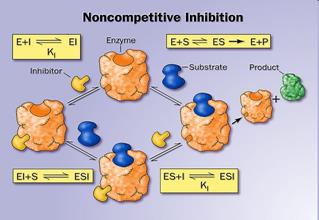
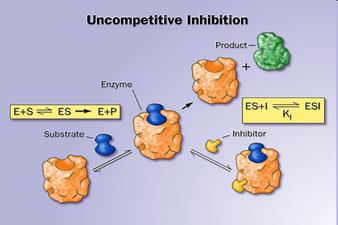
12. Activators and inhibitors of enzymatic reactions. Types of inhibition. (competitive, non-competitive, uncompetitive).
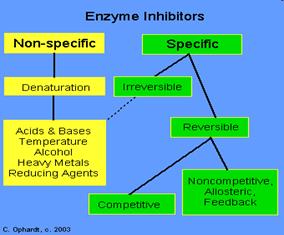
Inhibitors are chemicals that reduce the rate of enzymic reactions. The are usually specific and they work at low concentrations. They block the enzyme but they do not usually destroy it. Many drugs and poisons are inhibitors of enzymes in the nervous system.
• 
• When the inhibitor is present it fits into its site and there is a conformational change in the enzyme molecule
• The enzyme’s molecular shape changes
• The active site of the enzyme changes
• The substrate cannot bind with the substrate
Irreversible inhibitors: Combine with the functional groups of the amino acids in the active site, irreversibly. Examples: nerve gases and pesticides, containing organophosphorus, combine with serine residues in the enzyme acetylcholine esterase.
Reversible inhibitors: These can be washed out of the solution of enzyme by dialysis.
There are two categories of inhibitors: 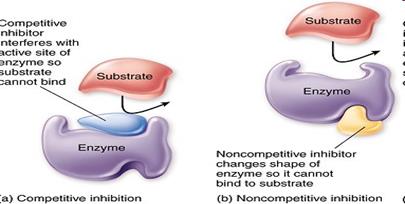
The effect of enzyme inhibition:
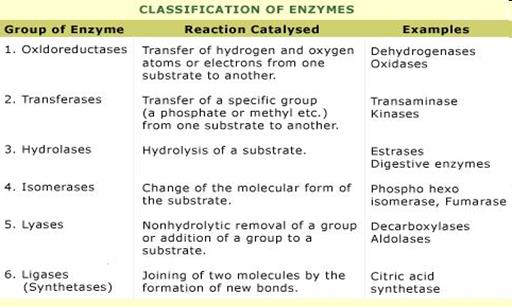
13. Enzyme classification.
14. Carbohydrates. Their distribution in nature. Functions of carbohydrates. Classification of carbohydrates. Most extended in nature monosaccharides (glucose, fructose, galactose).
Carbohydrates are carbon compounds that contain large quantities of hydroxyl groups. The simplest carbohydrates also contain either an aldehyde moiety (these are termed polyhydroxyaldehydes) or a ketone moiety (polyhydroxyketones). All carbohydrates can be classified as either monosaccharides, oligosaccharides or polysaccharides. Anywhere from two to ten monosaccharide units, linked by glycosidic bonds, make up an oligosaccharide. Polysaccharides are much larger, containing hundreds of monosaccharide units. The presence of the hydroxyl groups allows carbohydrates to interact with the aqueous environment and to participate in hydrogen bonding, both within and between chains. Derivatives of the carbohydrates can contain nitrogens, phosphates and sulfur compounds. Carbohydrates also can combine with lipid to form glycolipids or with protein to form glycoproteins
Carbohydrates have six major functions within the body:
| # Carbons | Category Name | Relevant examples |
| Triose | Glyceraldehyde, Dihydroxyacetone | |
| Tetrose | Erythrose | |
| Pentose | Ribose, Ribulose, Xylulose | |
| Hexose | Glucose, Galactose, Mannose, Fructose | |
| Heptose | Sedoheptulose | |
| Nonose | Neuraminic acid, also called sialic acid |
15. Isomerism of monosaccharides. Types of isomerism (D, L-isomers, α and β - anomers).
Due to the fact that carbohydrates contain multiple stereocenters, many isomers are possible including enantiomers, diastereoisomers, and epimers.
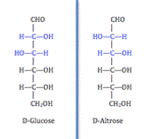
Two carbohydrates are said to be enantiomers if they are nonsuperimposable mirror images of one another. An example of an enantiomer is the D and L isomers of glucose, as shown by the figure to the right.
A second type of isomer seen in carbohydrates are diastereoisomers. Carbohydrates are classified as diastereomers if their chiral carbons are connected to the exactly the same substrates but connected at differing configurations (R or S). Unlike an enantiomer, diastereomers are NOT object and mirror image. An example of two carbohydrates that are diastereoisomers are D-Glucose and D-Altrose as seen in the figure to the left.
Lastly, another type of isomer that carhbohydrates that can take on are epimers. Epimers are two diastereomers that differ only at one stereocenter.[1] As shown in the figure below, D-Glucose and D-Mannose are an example of an epimer.
The two different forms of cyclic sugars, alpha and beta, are referred to as anomers. For example, in D -glucose, the hydroxy group on carbon 5 attacks the carbonyl carbon forming a six membered ring with the carbon that was attacked being known as the anomeric carbon. The resulting hemiacetal sugar is known as a pyranose. α - D -glucose is formed if the newly formed hydroxyl group is pointed in an opposite direction to the CH2OH group in Haworth projection, and β - D -glucose is formed if the hydroxyl group is pointed in the same direction as the CH2OH group. The majority, about 66% of D -glucose exist in β form because when the molecule is in chair conformation, all the bulky hydroxyl groups will be placed in equatorial position - which have lesser steric hindrance between the bulky groups. Thus, β - D -glucose is more stable than α - D -glucose that occupied typically 33% of D -glucose molecules, whereas the remaining 1% is in the open-chain form.
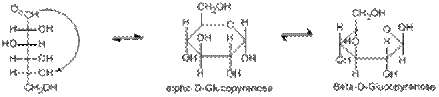
16. Reducing properties of monosaccharides. The formation of sugar acids.
The monosaccharides commonly found in humans are classified according to the number of carbons they contain in their backbone structures. The major monosaccharides contain four to six carbon atoms.
The most important chemical property of monosaccharides is the reducing power
The reducing power of monosaccharides comes from the hydroxyl group or aldehyde group. If it is an aldose, the aldehyde group gives the significant reducing power. Otherwise, it is the hydroxyl group.
When an aldose is oxidized, it would be turned into the corresponding acid. e.g.
Glucose -------- > gluconic acid
At the same time, the aldehyde group of the glucose is oxidized into the acid group.
If it is a ketose, the molecule would be cut into two parts from the location of the ketone group.
A reducing sugar is any sugar that either has an aldehyde group or is capable of forming one in solution through isomerism. The aldehyde functional group allows the sugar to act as a reducing agent, for example in the Tollens' test or Benedict's test, or the Maillard reaction, important in the browning of many foods. The cyclic hemiacetal forms of aldoses can open to reveal an aldehyde and certain ketoses can undergo tautomerization to become aldoses. However, acetals, including those found polysaccharide linkages, cannot easily become a free aldehyde.
Sugar acids are monosaccharides with a carboxyl group.[1]
Main classes of sugar acids include:
· Aldonic acids, in which the aldehyde functional group of an aldose is oxidized
· Ulosonic acids, in which the first hydroxyl group of a 2-ketose is oxidised creating an α-ketoacid.
· Uronic acids, in which the terminal hydroxyl group of an aldose or ketose is oxidized
· Aldaric acids, in which both ends of an aldose are oxidized
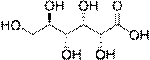
 Aldonic acid
Aldonic acid
| 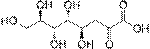
 Ulosonic acid
Ulosonic acid
| 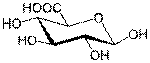
 Uronic acid
Uronic acid
| 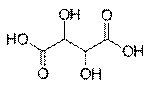
 Aldaric acid
Aldaric acid
|
17. Oligosaccharides, structural features (for example disaccharides). Reducing and non-reducing disaccharides. Examples. Lactose, sucrose, maltose.
oligosaccharide, any carbohydrate of from three to six units of simple sugars (monosaccharides). A large number of oligosaccharides have been prepared by partially breaking down more complex carbohydrates (polysaccharides). Most of the few naturally occurring oligosaccharides are found in plants. Raffinose, a trisaccharide found in many plants, consists of melibiose (galactose and glucose) and fructose. Another plant trisaccharide is gentianose. Maltotriose, a trisaccharide of glucose, occurs in some plants and in the blood of certain arthropods.
This section is divided into the reducing and non-reducing types of disaccharide. If two monosaccharides are linked through their anomeric centers the disaccharide formed is a non-reducing disaccharide. If one monosaccharide is linked by one of its other hydroxyl groups, then the anomeric center is unsubstituted and a reducing disaccharide occurs. Two monosaccharides have many more ways of connecting to form a reducing disaccharide than a non-reducing disaccharide.
| .Reducing Disaccharides | |
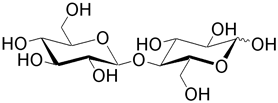
| Cellobiose (4- O -β-D-glucopyranosyl-D-glucose) results from the hydrolysis of cellulose by bacteria. Mammals lack the necessary enzymes—cellobiohydrolases and endo -cellulases—to hydrolyze cellulose. Cellobiose constitutes materials such as cotton and paper. Maltose is a homopolmyer of cellobiose. |
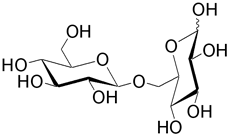
| Gentiobiose (6- O -β-D-glucopyranosyl-D-glucose) is found in many glycosides such as amygdalin. |
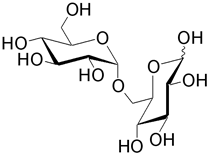
| Isomaltose (6- O -α-D-glucopyranosyl-D-glucose) is formed from two glucose monosaccharides. It is often found at the branching points of amylopectin and glycogen. |
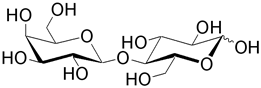
| Lactose (4- O -β-D-galactopyranosyl-D-glucose) is the predominant disaccharide found in milk. Lactose intolerance is a condition in which there is a lack of the enzyme lactase. Galactosaemia is a condition that results from an inability to process the D-galactose after hydrolysis. |
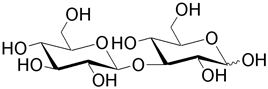
| Laminaribiose is a polysaccharide building unit for laminarin (brown algae), pachyman (fungi), paramylon (unicellular algae), and callose. |
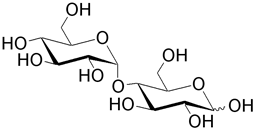
| Maltose (4- O -α-D-glucopyranosyl-D-glucose) results from hydrolysis of starch by enzymes (amylases) in the mammalian digestive tract. Notice that the glycosidic linkage is α and that of the homopolymer cellobiose is a β linkage. Maltose is used as a sweetner and as a substrate for fermentation. It is also the constituent of the polymer amylose. |
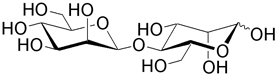
| Mannobiose is the unit for the plant polysaccharide mannan. |
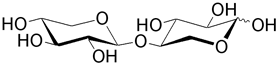
| Xylobiose is the unit found in many polysaccharides such as the xylans that constitute plant cell walls. |
| Non-Reducing Disaccharides | |
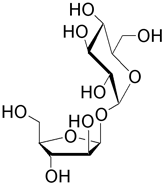
| Sucrose (β-D-fructofuranosyl α-D-glucopyranoside) is the predominant disaccharide found in sugar cane and sugar beet. It is a well known sweetner and has a five-membered furanosyl unit. 108 tons of sucrose are produced annually for consumption. |
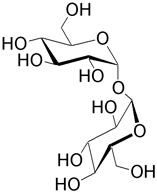
| Trehalose (α-D-glucopyranosyl α-D-glucopyranoside) is found in microbes, fungi, and certain insects. One isomer is neotrehalose with an α,β link. Another isomer is isotrehalose which has a β,β link. |
18. Polysaccharides. Structure, properties. Starch, glycogen, cellulose.
Most of the carbohydrates found in nature occur in the form of high molecular weight polymers called polysaccharides. The monomeric building blocks used to generate polysaccharides can be varied; in all cases, however, the predominant monosaccharide found in polysaccharides is D-glucose. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Polysaccharides composed of more than one type of monosaccharide are termed heteropolysaccharides
Natural saccharides are generally of simple carbohydrates called monosaccharides with general formula (CH2O) n where n is three or more. A typical monosaccharide has the structure H-(CHOH) x (C=O)-(CHOH) y -H, that is, an aldehyde or ketone with many hydroxyl groups added, usually one on each carbon atom that is not part of the aldehyde or ketone functional group. Examples of monosaccharides are glucose, fructose, and glyceraldehyde[5]


Amylose is a linear polymer of glucose mainly linked with α(1→4) bonds. It can be made of several thousands of glucose units. It is one of the two components of starch, the other being amylopectin.
Polysaccharides are composed of long chains of monosaccharide units bound together by glycosidic bonds. Polysaccharides contain more than ten monosaccharide units. Definitions of how large a carbohydrate must be to fall into the categories polysaccharides or oligosaccharides vary according to personal opinion.
Polysaccharides are an important class of biological polymers. Their function in living organisms is usually either structure- or storage-related. Starch (a polymer of glucose) is used as a storage polysaccharide in plants, being found in the form of bothamylose and the branched amylopectin. In animals, the structurally similar glucose polymer is the more densely branchedglycogen, sometimes called 'animal starch'. Glycogen's properties allow it to be metabolized more quickly, which suits the active lives of moving animals.
Cellulose and chitin are examples of structural polysaccharides. Cellulose is used in the cell walls of plants and other organisms, and is said to be the most abundant organic molecule on earth.[6] It has many uses such as a significant role in the paper and textile industries, and is used as a feedstock for the production of rayon (via the viscose process), cellulose acetate, celluloid, and nitrocellulose. Chitin has a similar structure, but has nitrogen-containing side branches, increasing its strength. It is found in arthropod exoskeletons and in the cell walls of some fungi. It also has multiple uses, including surgical threads.
Polysaccharides are common sources of energy. Many organisms can easily break down starches into glucose, however, most organisms cannot metabolize cellulose or other polysaccharides like chitin and arabinoxylans. These carbohydrates types can be metabolized by some bacteria and protists. Ruminants and termites, for example, use microorganisms to process cellulose.
Even though these complex carbohydrates are not very digestible, they may comprise important dietary elements for humans. Called dietary fiber, these carbohydrates enhance digestion among other benefits. The main action of dietary fiber is to change the nature of the contents of the gastrointestinal tract, and to change how other nutrients and chemicals are absorbed.[7][8] Soluble fiber binds to bile acids in the small intestine, making them less likely to enter the body; this in turn lowers cholesterol levels in the blood.[9] Soluble fiber also attenuates the absorption of sugar, reduces sugar response after eating, normalizes blood lipid levels and, once fermented in the colon, produces short-chain fatty acids as byproducts with wide-ranging physiological activities (discussion below). Although insoluble fiber is associated with reduced diabetes risk, the mechanism by which this occurs is unknown
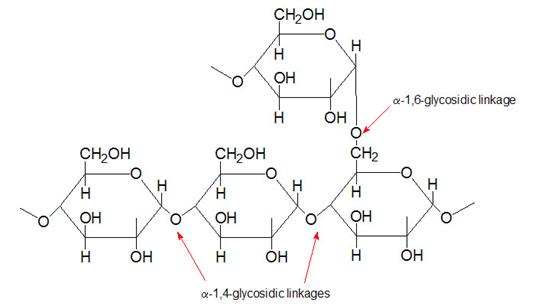
Glycogen
Glycogen is the major form of stored carbohydrate in animals. This crucial molecule is a homopolymer of glucose in α–(1,4) linkage; it is also highly branched, with α–(1,6) branch linkages occurring every 8-10 residues. Glycogen is a very compact structure that results from the coiling of the polymer chains. This compactness allows large amounts of carbon energy to be stored in a small volume, with little effect on cellular osmolarity.
Дата добавления: 2015-10-31; просмотров: 334 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Positively-charged amino acids | | | Write questions to which the words in bold are the answers. |