
|
Читайте также: |
Fe2O3 + H2 =
Fe + O2 =
Fe2O3 + CO =
Fe + Cl2 =
Fe + HNO3 =
Fe(OH)2 + O2 + H2O =
FeSO4 + KMnO4 + H2SO4 =
Fe2O3 + Na2CO3 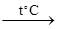
FeCl3 + Cu =
FeCl3 + KI =
FeCl3 + K4[Fe (CN)6] =
FeCl2 + K3[Fe (CN)6] =
FeCl3 + KCNS =
Na2FeO4 + H2SO4 =
Na2FeO4 + NH4OH =
Co + HNO3 =
Co + O2 =
Ni + O2 =
Co(OH)2 + O2 + H2O =
CoCl2 + Br2 + NaOH =
Ni(OH)3 + HCl =
Co(OH)3 + H2SO4 =
Co(NO3)2 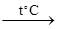
NiSO4 + NH3 =
9. The sum of coefficients of the equation of the reaction of cobalt (ІІ) hydroxide with water solution of hypobromite of sodium is equal to:
А. 6
В. 7
С. 9
D. 14
Е. 18
10. The sum of coefficients of the equation of the reaction of hydroxide of nickel with chlorine in the medium of hydroxide of sodium is equal to:
А. 6
В. 7
С. 9
D. 13
Е. 17
11. The sum of coefficients of the equation of the reaction of sulfate of iron (ІІ) with a solution of potassium permanganate acidified by sulfuric acid is equal to:
А. 24
В. 28
С. 36
D. 43
Е. 49
12. The sum of atoms of oxygen of the equation of the reaction of sulfate of iron (ІІ) with a solution of potassium permanganate acidified by sulfuric acid is equal to:
А. 56
В. 72
С. 80
D. 98
Е. 114
20. The sum of atoms of oxygen of the equation of the reaction of hydroxide of nickel (ІІІ) with muriatic acid is equal to:
А. 12
В. 34
С. 56
D. 68
Е. 72
31. The sum of coefficients of the equation of the reaction of water solutions of chloride of iron (ІІІ) and soda is equal to:
А. 16
В. 19
С. 22
D. 26
Е. 31
32. The sum of atoms of oxygen of the equation of the reaction of water solutions of chloride of iron (ІІІ) and soda is equal to:
А. 12
В. 18
С. 24
D. 38
Е. 56
Дата добавления: 2015-07-25; просмотров: 53 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| B. Fe(OH)Cl | | | Chemical properties of iron |