
|
Читайте также: |
Iron(III) readily forms octahedral complexes. The hexaaquo-ion [Fe(H2O)6]3+ is found only in a few solid hydrated salts (or in their acidified solutions), for example Fe2(SO4)3.9H2O, Fe(ClO4)3.10H2O. In many other salts, the anion may form a complex with the iron(III) and produce a consequent colour change. For example, iron(III) chloride hydrate or solution. Stable anionic complexes are formed with a number of ions, for example with oxalate, C2O42–, and cyanide. The redox potential of the iron(II) – iron (III) system is altered by complex formation with each of these ligands; indeed, the hexacyanoferrate(III) ion, [Fe(CN)6]3–, is most readily obtained by oxidation of the corresponding iron(II) complex, because
[Fe(H2O)6]3+ + e – 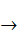 [Fe(H2O)6]2+, E0 = +0.77 V,
[Fe(H2O)6]2+, E0 = +0.77 V,
[Fe(CN)6]3– + e – 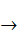 [Fe(CN)6]4–, E0 = +0.36 V.
[Fe(CN)6]4–, E0 = +0.36 V.
The thiocyanate ion SCN- forms an intensely red-coloured complex (most simply represented as [Fe(SCN)(H2O)5]2+) which is a test for iron(III). However, unlike cobalt(III), iron(III) does not form stable hexammines in aqueous solution, although salts containing the ion [Fe(NH3)6]3+ can be obtained by dissolving anhydrous iron(III) salts in liquid ammonia.
As for the +3 state, iron(II) forms a variety of complexes which are usually 6-coordinate and octahedral. Replacement of the water ligands in green [Fe(H2O)6]2+ by ammonia molecules is incomplete in aqueous ammonia, but reaction of the anhydrous chloride with gaseous or liquid ammonia gives the complex [Fe(NH3)6]Cl2. The water ligands are more easily replaced by cyanide ions to give the hexacyanoferrate(II) ion, [Fe(CN)6]4– Many salts of this ion are known, for example, the soluble yellow hydrate K4[Fe(CN)6].3H2O, and the insoluble brown copper(II) salt Cu2[Fe(CN)6].
The reaction between aqueous Fe3+ ions and [Fe(CN)6]4– yields an intense blue precipitate, prussian blue, which is iron(III) hexacyanoferrate(II), Fe4[Fe(CN)6]3; the same material, called Turnbull’s blue is obtained by addition of Fe2+ (aq.) ions to [Fe(CN)6]3+ ions. The intense colour of this compound is due to charge-transfer. The formation of [Fe(CN)6]4– ions causes the iron(II) to change its properties (for example it is not precipitated as the hydroxide with alkali or as the sulfide with S2- ions); it is more readily oxidised to the +3 state, since
[Fe(CN)6]3 – (aq) + e– 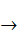 [Fe(CN)6]4–(aq); E0 = +0.36 V,
[Fe(CN)6]4–(aq); E0 = +0.36 V,
When concentrated sulfuric acid is added to a nitrate in the presence of aqueous iron(II) sulfate, the liberated nitrogen oxide forms a brown complex [Fe(H2O)5NO]2+ which appears as a "brown ring' at the acid-aqueous interface.
Perhaps the most important complex of iron(II) is heme (or haeme). Haemoglobin, the iron-containing constituent of the blood, consists essentially of a protein, globin, attached through a nitrogen atom at one coordination position of an octahedral complex of iron(II). Of the other five coordination positions, four (in a plane) are occupied by nitrogen atoms, each of which is part of an organic ring system—the whole system is a porphin. The sixth position (see Figure below), is occupied either by an oxygen molecule or a water molecule, and here reversible oxygen uptake can occur, as shown, thereby enabling oxygen to be transported from one part of the body to another. Coordination of a ligand CN or CO instead of water prevents this process, and the toxicity of cyanide or carbon monoxide is, in part due to this fact.

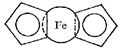
Fe(ІІ), Co(ІІ), and Ni(ІІ) form very interesting coordination compounds with cyclopentadienyl, С5Н5. The first compound prepared was the compound of Fe(ІІ) by the reaction:
FeCl2 + 2NaC5H5 ® Fe(C5H5)2 + 2NaCl,
that was performed in diethylether medium. This compound is called ferrocene.
|
The atom of iron in this compound is situated between two planes formed by С5Н5- groupings. Classical d-bonds of a central atom with donor atoms of ligands are absent in this case.
The general name metallocene is derived from ferrocene, (C5H5)2Fe or Cp2Fe, systematically named bis(η5-cyclopentadienyl)iron(II). Thus, metallocenes are sometimes referred to as sandwich compounds.
Дата добавления: 2015-07-25; просмотров: 62 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Cobalt (III) compounds | | | COMPLEXES OF COBALT |