
|
Читайте также: |
Hydrides. Their composition depends on the ratio of components (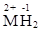 — the lack of hydrogen, H2) and like hydrides of s-elements they can be decomposed by water and oxidised by O2:
— the lack of hydrogen, H2) and like hydrides of s-elements they can be decomposed by water and oxidised by O2:
2 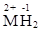 + 6
+ 6  = 2
= 2  + 5
+ 5 
 + 3
+ 3  =
=  + 3
+ 3 
Oxides. The examples of their preparation are shown below:
4Sc + 3O2 = 2Sc2О3
4Y(NO3)3 = 2Y2О3 + 12NO2 + 3O2
They react actively with water. In the series Sc —Y— La—Ac the basic properties of oxides are increased, as well as the rate of their reaction with water,? exothermic heat effect of the reaction.
Hydroxides. In the series Sc(OH)3—Y(OH)3—La(OH)3—Ac(OH)3 basic properties are also increased. This fact is explained by the M3+ size growth and weakening the bond of these ions with hydroxide groups ОН-. Sc(OH)3 is an amphoteric hydroxide:
Sc(OH)3 + 3NaOH = Na3[Sc(OH)6]
Oxoscandates are formed when melting Sc2O3 or Sc(OH)3 with alkalis:
Sc2O3 + 2NaOH = 2NaScO2 + H2O
Y(OH)3, La(OH)3 have low solubility in water, although their solutions form weak alkaline medium. For instance, saturated solution of La(OH)3 absorbs CO2 from air:
2La(OH)3 + 3CO2 ® La2(CO3)3 + 3H2O
Salts. Solubility of them is close to alkali-earth metals salts solubility. Fluorides MF3, carbonates М2(СО3)3, phosphates МРО4 and sulfates M2(SO4)3 are soluble in water.
Scandium forms the most stable coordination compounds with coordination number 6. After scandium it becomes larger and stability of a complex ion decreases. For instance, there are the following aquacomplexes of the Sc subgroup elements: [Sc(H2O)6]3+, [Y(H2O)9]3+, [La(H2O)9]3+. ScF3 can react with fluorides of alkali metals and form soluble complexes:
ScF3 + 3KF = K3[ScF6]
Carbonates M2(СO3)3 dissolve in saturated carbonates of alkali metals (or ammonium) due to the double salt formation:
K2CO3 + La2(CO3)3 ® 2K[La(CO3)2]
Halides can be crystalhydrates. Losing water after thermal decomposition, they are transformed to oxohalides:
ScCl3.6H2O  ScCl3.3H2O
ScCl3.3H2O  Sc(OH)2Cl
Sc(OH)2Cl  ScOCl
ScOCl
- H2O - H2O,-HCl - H2O
Дата добавления: 2015-07-25; просмотров: 79 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Vanadium Subgroup | | | Titanium subgroup elements, IVB |