
|
Читайте также: |
Metallic scandium was obtained in 1937 by electrolysis of a eutectic mixture of potassium, lithium, and scandium chlorides at 700–800 °C. Especially pure 99% metallic scandium was produced in 1960. Aluminium-scandium alloys are widely used.
Metallic Ti, Zr and Hf preparation has difficulties since the bond energy of these elements with O, N, and C is high. For instance, the reagent required to extract titanium from its various ores can not be carbon, because with titanium the latter gives titanium carbide.
Titanium subgroup elements are mostly produced by metalthermy from chlorides or fluorocomplexes. W. Kroll proved that titanium could be produced by reducing titanium tetrachloride (TiCl4) with magnesium, calcium, and even sodium in what became known as the Kroll process:
Titanium chloride is obtained at the action of carbon and chlorine on titanium ores:
TiO2 + 2C + 2Cl2 = TiCl4 + 2CO
It is reduced by magnesium:
TiCl4 + 2Mg = Ti + 2MgCl2
The product is spongy titanium.
Production of Zr and Hf is the reaction between fluorocomplexes of metals with sodium:
K2ZrF6 + 4Na = Zr + 4NaF + 2KCl
In turn, fluorocomplexes are obtained by sintering silicates:
ZrSiO4 + K2SiF6 = K2ZrF6 + 2SiO2
Titanium of very high purity was obtained in small quantities when Anton Eduard van Arkel and Jan Hendrik de Boer discovered the iodide, or crystal bar, process in 1925, by reacting with iodine and decomposing the formed vapors over a hot filament to pure metal.
Zr(cryst) + 2I2 (g) 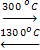 ZrI4(cryst)
ZrI4(cryst)
Equilibrium of this reaction is shifted to the left at high temperature, and to the right at low temperature (DS<0). This process is usually called the transport reaction and can be applied to other elements (Si, Bi etc.)

A titanium crystal bar made by the iodide process
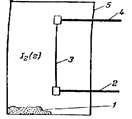
The scheme of transport reaction: 1- dusty metal, 2, 4 – current supply, 3 – Zr of very high purity that is heated, 5 - chamber.
Дата добавления: 2015-07-25; просмотров: 67 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Vanadium Subgroup | | | Vanadium Subgroup |