
|
Читайте также: |
These are compounds with a functional group that has nitrogen atoms in it, which includes amines, amides.
Amines 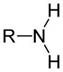
Amines are organic compounds that are derivatives of ammonia (NH3), in which the hydrogen atoms of ammonia have been replaced by alkyl or aryl groups. Amines can be identified by the presence of a nitrogen atom with a lone pair of electrons. Amines that have an aromatic ring attached to the nitrogen atom are known as aromatic amines, while others are known as aliphatic amines. All amines are more basic, when compared to ammonia. Amines can be categorized into three classes, and these are as follows:
Primary Amines
In primary amines, one of the three hydrogen atoms in ammonia, is replaced by an alkyl or aryl group. Thus, primary amines can be aliphatic or aromatic. The general chemical formula for primary amines is RNH2, and they are named as "alkylamine". Examples of aliphatic primary amines are methylamine, ethylamine, and propylamine, while an example of aromatic primary amines is phenylamine (aniline).
Secondary Amines
In secondary amines, two of the three hydrogen atoms in ammonia, are replaced by alkyl or aryl groups. The general formula for secondary amines is R2NH, and they are named as "dialkylamine". Secondary amines can be cyclic. Examples of secondary aliphatic amines are dimethylamine, ethylmethylamine, and diethylamine, while an example of secondary aromatic amines is diphenylamine.
Tertiary Amines
In tertiary amines, all the three hydrogen atoms of ammonia, are replaced by alkyl or aryl groups. In other words, a tertiary amine has three hydrocarbon groups attached to the nitrogen atom. Tertiary amines can be cyclic. The general formula for tertiary amines is R3N, and they are named as "trialkylamine". Examples are trimethylamine and triphenylamine.
If all four hydrogen atoms of an ammonium ion are replaced with alkyl or aryl groups, then a quaternary ammonium salt is formed, which is ionic. The simplest example of such a salt is tetramethylammonium chloride (CH3)4N+ Cl-. Given below are a few examples of primary, secondary, and tertiary amines.
Дата добавления: 2015-08-27; просмотров: 62 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Examples | | | Examples |