
|
Читайте также: |
Free Manganese And Compounds Mn(0)
Chemical activity of elements in the series Mn-Tc-Re sharply decreases.
Manganese is between aluminium and zinc in the electromotive series: the standard electrode potential of the system Mn2+/Mn is—1.179 V. It is chemically active, and decomposes cold water slowly.
Mn +2HCI = MnCl2 + H2
5Mn + 12HNO3(dil.) 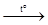 5Mn(NO3)2 + N2 + 6H2O.
5Mn(NO3)2 + N2 + 6H2O.
Manganese dissolves readily in dilute acids to give manganese(II) salts. It dissolves in dilute hydrochloric and nitric acids, and also in hot concentrated sulfuric acid (it is virtually insoluble in cold sulfuric acid), forming Mn2+ cations. Tc and Re interact only with oxidant acids:
3E + 7HNO3 = 3HEO4 + 7NO + 2H2O
In the air, manganese is covered with a thin oxide film preventing its further oxidation even when heated, however powdered manganese is oxidized quite readily.
Mn actively reacts with chlorine and fluorine (MnHal2), sulfur (MnS, MnS2) and when heating with B, C, Br, I. Rhenium forms ReCI5 ReF6 ReS2 and Re2S7. The latter compound can be considered as thioanhydride. It can be easily dissolved in sulfides of alkali metals:
Na2S + Tc2S7 = NaTcS4

Дата добавления: 2015-07-25; просмотров: 101 | Нарушение авторских прав
| <== предыдущая страница | | | следующая страница ==> |
| Occurrence | | | Scheme of chemical properties of manganese |